A method for recycling and treating waste pickling liquid of stainless steel containing iron, chromium and nickel
A technology for recycling and treating pickling waste liquid, which is applied in chemical instruments and methods, water/sewage multi-stage treatment, water/sludge/sewage treatment, etc., can solve the problems of pickling waste liquid environmental pollution and achieve ecological protection Environment, the effect of creating economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
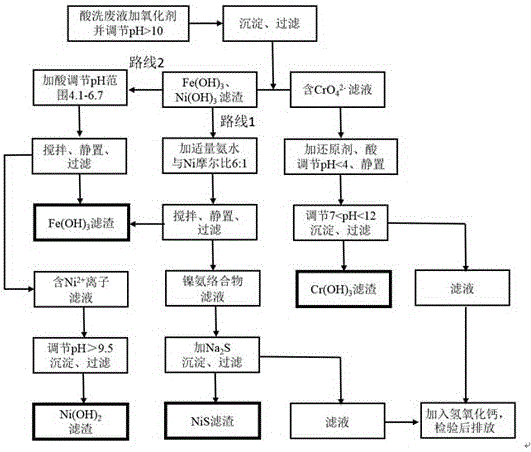
[0031] Get stainless steel pickling waste liquid 100mL and pour in the 500mL beaker, measure the concentration of iron ion through flame atomic absorption spectrophotometry about 83mg / L, chromium (III) ion concentration is 23mg / L, and nickel ion concentration is 21mg / L.
[0032] First, add 10 mL of oxidant sodium hypochlorite solution to the pickling waste liquid and add 2 mol / L sodium hydroxide solution to adjust the pH value to 10.5. After the precipitation is complete, filter, and the chromium element is completely oxidized to hexavalent chromate (CrO4 2- ) exists in the filtrate 1, and the filter residue 1 is to be treated for the subsequent separation of iron and nickel. Use 2mol / L sulfuric acid solution to adjust the pH value of the filtrate 1 to 3.5, add the reducing agent sodium sulfite, let it stand for half an hour, then use sodium hydroxide solution to adjust the pH value back to alkaline 10.3, and filter after the precipitation is complete to obtain Cr (OH) 3 The ...
Embodiment 2
[0036] Get stainless steel pickling waste liquid 100mL and pour in the 500mL beaker, measure the concentration of iron ion through flame atomic absorption spectrophotometry to be about 98mg / L, chromium (III) ion concentration is 27mg / L, and nickel ion concentration is 27mg / L. Compared with embodiment 1, according to the pH adjustment range in the dosage example 2 of the sulfuric acid that adds and sodium hydroxide solution is different.
[0037] First, add 12mL of oxidant sodium hypochlorite solution to the pickling waste liquid and add 2 mol / L sodium hydroxide solution to adjust the pH value to 11.2. After the precipitation is complete, filter, and the chromium element is completely oxidized to hexavalent chromate (CrO4 2- ) exists in the filtrate 1, and the filter residue 1 is to be treated for the subsequent separation of iron and nickel. Use 2mol / L sulfuric acid solution to adjust the pH value of the filtrate 1 to 2.2, add the reducing agent sodium sulfite, let it stand fo...
Embodiment 3
[0041] Get stainless steel pickling waste liquid 100mL and pour in the 500mL beaker, measure the concentration of iron ion through flame atomic absorption spectrophotometry to be about 91mg / L, chromium (III) ion concentration is 23mg / L, and nickel ion concentration is 27mg / L. Compared with Examples 1 and 2, the method for separating iron and nickel is route 2-fractional precipitation.
[0042] First, add 12 mL of oxidant sodium hypochlorite solution to the pickling waste liquid and add 2 mol / L sodium hydroxide solution to adjust the pH value to 11.5. After the precipitation is complete, filter, and the chromium element is completely oxidized to hexavalent chromate (CrO4 2- ) exists in the filtrate 1, and the filter residue 1 is to be treated for the subsequent separation of iron and nickel. Use 2mol / L sulfuric acid solution to adjust the pH value of the filtrate 1 to 3.1, add the reducing agent sodium sulfite, let it stand for half an hour, then use sodium hydroxide solution t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com