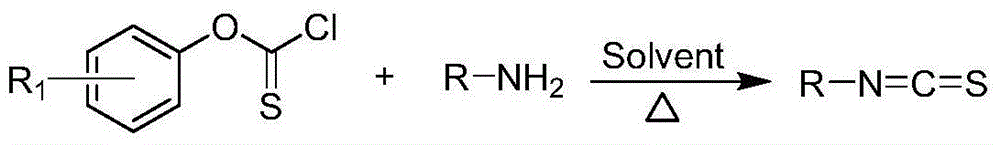Alkali-free green synthetic method for isothiocyanate
An isothiocyanate, green synthesis technology, applied in the direction of organic chemistry, etc., to achieve the effect of good application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Phenoxythiol chloride (1 mmol), 15 ml of toluene and aniline (2 mmol) were successively added into a 50 ml one-necked flask, and heated and stirred at 115° C. for 16 h. Stop the reaction, cool down, spin dry, add 20ml of dichloromethane, then use 10% dilute hydrochloric acid to extract and separate the organic phase, wash with water, wash with saturated brine, anhydrous Na 2 SO 4 Dry and separate by column chromatography (ethyl acetate:petroleum ether=1:5) to obtain phenyl isothiocyanate with a yield of 80%.
[0026] 1 H NMR (500MHz, CDCl 3 )δ: 7.35(t, J=7.5Hz, 2H, ArH), 7.28(d, J=7.5Hz, 1H, ArH), 7.23(d, J=7.5Hz, 2H, ArH); 13 C NMR (125MHz, CDCl 3 )δ: 130.2, 128.5, 126.3, 124.7, 123.2; MS (70eV) m / z (%): 135 (M + ,100).
Embodiment 2
[0028] Referring to the method of Example 1, using p-fluorophenoxysulfonyl chloride as the carbon sulfide reagent, the yield of the target product was 78%.
Embodiment 3
[0030] Referring to the method of Example 1, the molar ratio of phenoxysulfonyl chloride to benzylamine was 1:1.2, and the yield of the target product was 61%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap