Compound for immunotherapy, and composition
A technology of compounds, chemical bonds, applied in the field of compounds for targeted immunotherapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0351] Compound preparation
[0352] In general, activated moieties represented by structures of general formula (I) can be prepared using the synthetic steps outlined below. In step (1), 4-chloro-3-nitroquinoline of general formula A and general formula R 1 NH 2 The reaction of amines generates 3-nitroquinolin-4-amines of general formula B. In step 2, 3-nitroquinolin-4-amines of general formula B are reduced to yield quinoline-3-4-diamines of general formula C. In step 3, quinoline-3-4-diamine of general formula C is reacted with carboxylic acid or its equivalent to generate 1H-imidazo[4,5c]quinoline of general formula D.
[0353]
[0354] Alternatively, compounds of general formula (I) can be prepared according to the synthetic methods described in US6,331,539B1, US6,451,810B1, US7,157,452 and US7,301027B2.
[0355] On the other hand, compounds of general formula (Ia) and general formula (Ib) can be prepared by using a linker to link both the targeting moiety and th...
Embodiment 1
[0419] Generation of HER2 or EGFR transfected L cell lines
[0420] Reagents: L cells from ATCC (Manassas, VA; Cat No. CRL2648), Her2 / pEZ-Lv105 or egfr / pCMV purchased from Sino Biological Inc., (Cat No. H10004) or GeneCopoeia, (Cat No. Z2866) cDNA, glucose DMEM, L-glutamine, Lipofectamine 2000 (Invitrogen; Carlsbad, CA).
[0421] To generate cell lines for screening conjugated trastuzumab, L cells expressing tagged Her2 or EGFR were generated. Her2 / pEZ-Lv105 or egfr / pCMV cDNA constructs were transfected into L cells (the cells were grown in high concentration glucose DMEM+10%FBS+2mM L-glutamine) by standard Lipofectamine2000 operating procedures.
[0422] Binding analysis (FACS analysis)
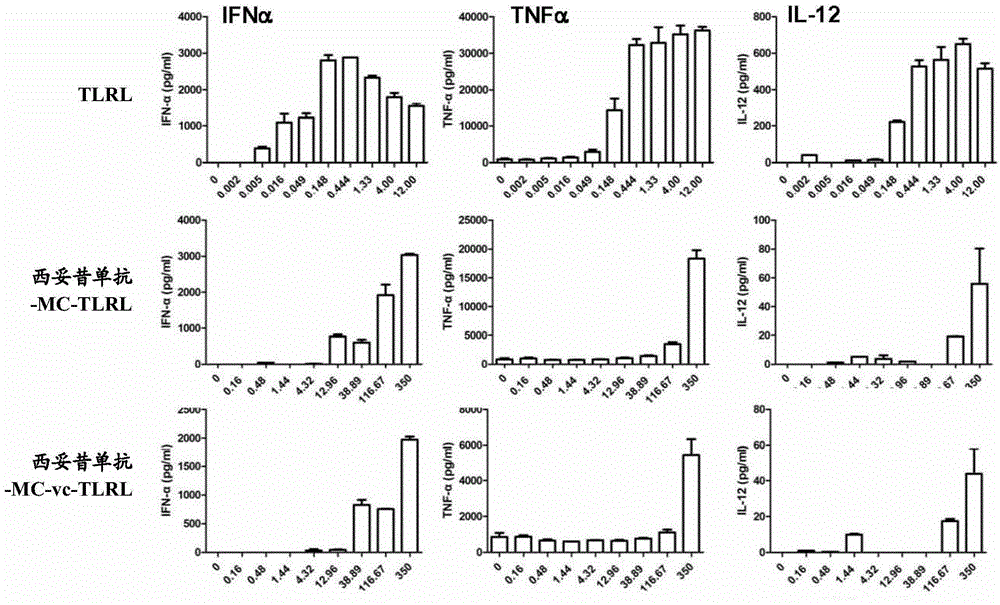
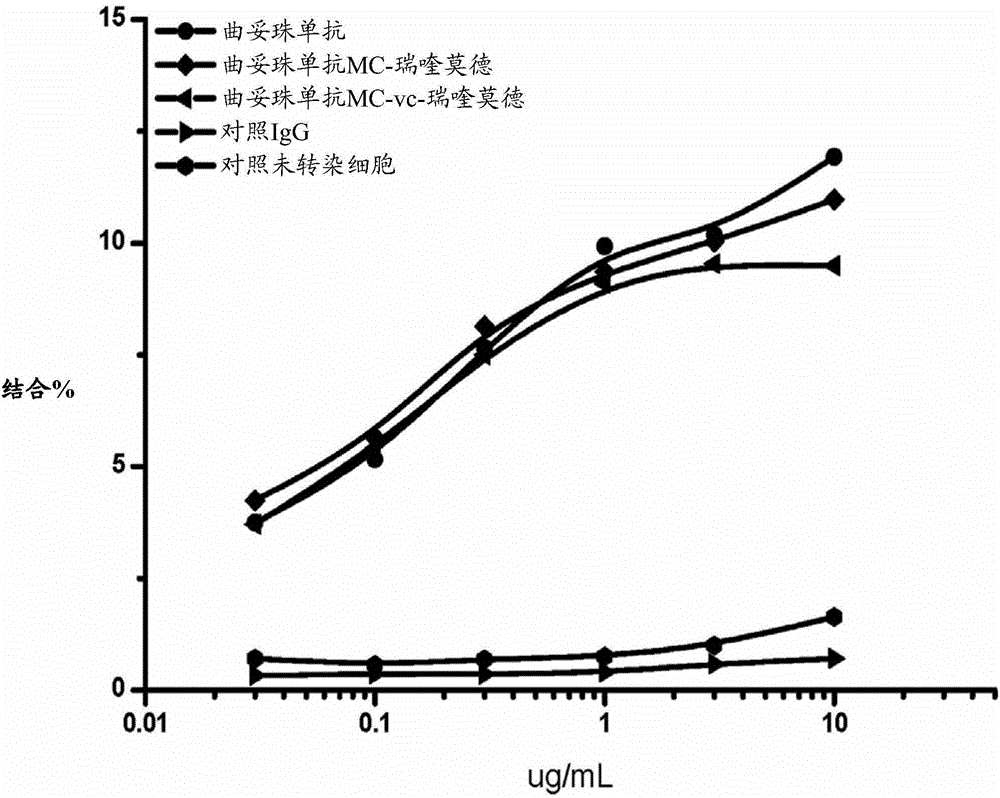
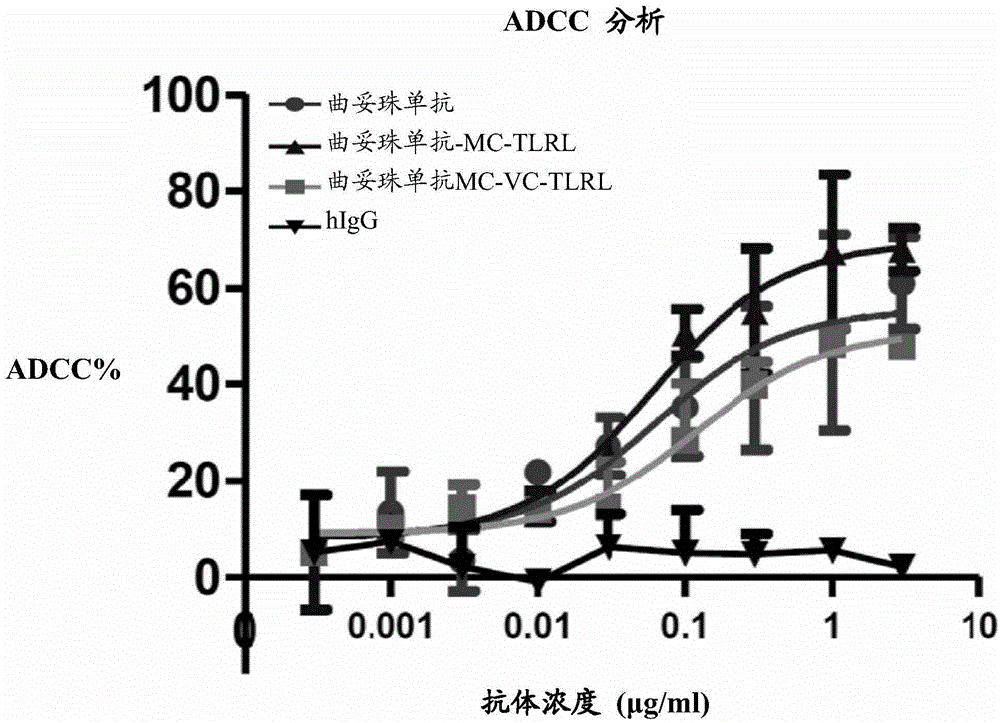
[0423]To determine the binding capacity of conjugated trastuzumab, FACS analysis was performed on L cells expressing human her2. Briefly, 100 μl of approximately 10 6 L cells with transiently transfected her2 were incubated with varying amounts of trastuzumab-conjugated antibodies, u...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com