Targeted nano-photo drug for photodynamic therapy of cancer
A photodynamic therapy and targeting technology, applied in drug combination, drug delivery, preparations for in vivo tests, etc., can solve the problem of limiting the total dose of active oxygen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Embodiment 1: with Ce 6 Preparation of Nanophotomedicine NPM-1 as Photosensitizer
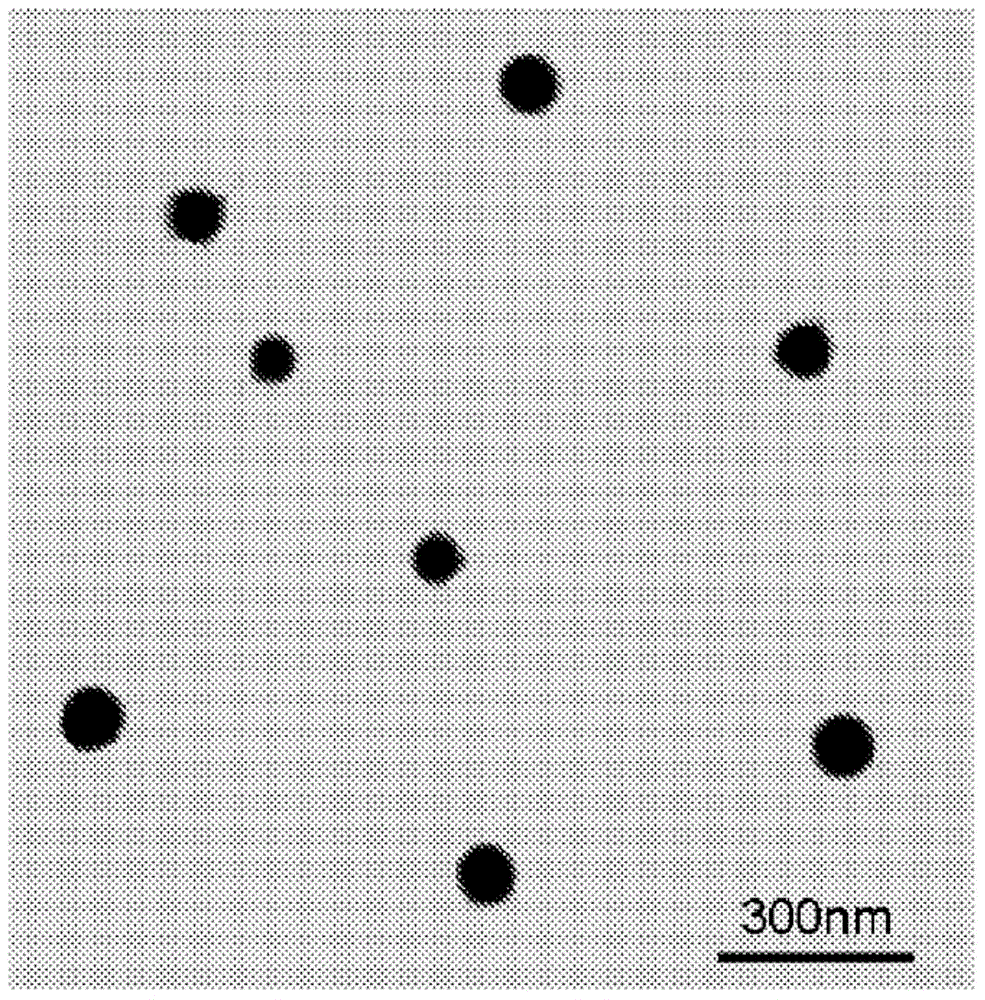
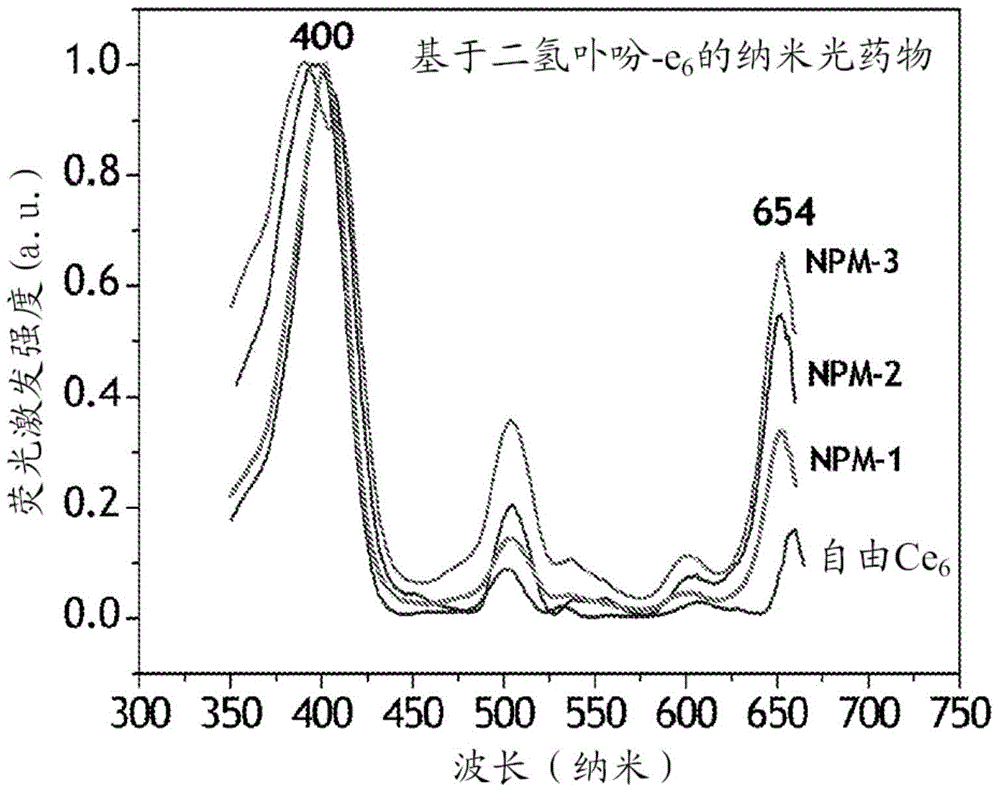
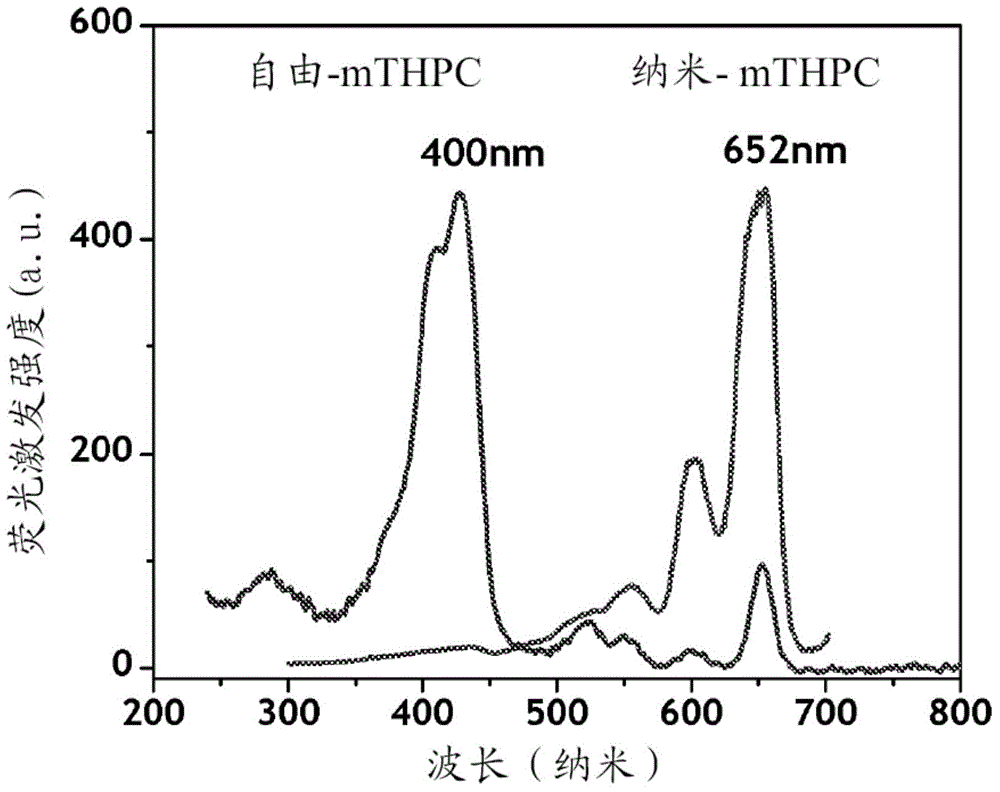
[0100] In this example, the photosensitizer chlorin e 6 (Ce 6 ) of nanophotomedicine (i.e., NPM-1), the optical absorption of the final structure of the nanophotomedicine in the Q-band (654nm) region is free Ce 6 about 3 times.
[0101] Ce at a concentration of 1 μM in 5 ml of 99% DMSO 6 (commercially available from, eg, Porphyrin Products (Logan, Utah)) was reacted with a 10-15 fold molar excess of EDAC and a 10-15 molar excess of sulfo-NHS. After reacting for 4 hours, the combined product was purified by gel filtration to obtain an ammonia-reactive photosensitizer, which was further reacted with 200 μL of silane coupling agent APTS. The coupling reaction is carried out in the dark at room temperature for 3 to 4 hours to obtain the compound Ce 6 -APTS. In the next step, in 10 ml of 99% ethanol medium, Ce 6 - APTS was reacted with 600 μL (about 600 mg) of TEOS or TMOS for 2-3...
Embodiment 2
[0103] Example 2: With Ce 6 Characteristics of Nanophotopharmaceutical NPM-2 as Photosensitizer
[0104] In this example, the ratio of Q-band light absorption to free Ce 6 Increased nanophotomedicine (NPM-2) process by about 4 times.
[0105] Ce at a concentration of 1 μM in 5 ml of 99% ethanol 6 React with 10-15 times molar excess of EDAC and 10-15 molar excess of sulfo-NHS. After about 4 hours of reaction, the conjugate was purified by gel filtration to obtain an ammonia-reactive photosensitizer, which was reacted with 300 μL of silane coupling agent APTS. The coupling reaction is carried out in the dark at room temperature for 3 to 4 hours to obtain the compound Ce 6 -APTS. In the next step, in 10 ml of 99% ethanol medium, Ce 6 -APTS was reacted with 800 μL of TEOS or TMOS for 3 hours to generate the precursor of NPM-2. Add 3ml of water and 600μL of NH 4 o 4 , at intervals of 2 minutes, ultrasonic treatment was performed for 15 minutes to hydrolyze the precur...
Embodiment 3
[0106] Embodiment 3: with Ce 6 Preparation of Nanophotomedicine NPM-3 as Photosensitizer
[0107] In yet another embodiment, the absorption ratio in the Q-band region is illustrated for free Ce 6 Increased preparation of nanophotomedicine (NPM-3) by approximately 7-fold.
[0108] Ce at a concentration of 1 μM in 5 ml of 99% ethanol 6 React with 10-15 times molar excess of EDAC and 10-15 molar excess of sulfo-NHS. After 4 hours of reaction, the combined product was purified by gel filtration to obtain ammonia-reactive Ce 6 , ammonia-reactive Ce 6 React with 600 μL of silane coupling reagent APTS. The coupling reaction is carried out in the dark at room temperature for 3 to 4 hours to obtain the compound Ce 6 -APTS. In the next step, in 10 ml of 99% ethanol medium, Ce 6 -APTS was reacted with 1000 μL of TEOS or TMOS for 2-3 hours to form a precursor of silane-coupled quasi-aggregated photopharmaceuticals. Add 3ml of water and 800μL of NH 4 o 4 , sonicated for 20...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap