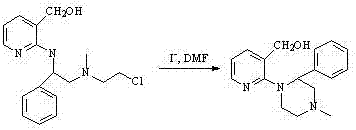
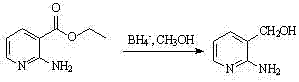A kind of synthetic method of Mitazenol
A synthetic method, the technology of mirazol, applied in the direction of organic chemistry, etc., can solve the problems of hindering the ring-closing reaction, many impurities in the reaction, and difficult ring-closing, etc., so as to ensure the yield and stability, the safety of the process route, and the reaction product high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]Add 3g of ethyl 2-aminonicotinate (0.0181mol) and 50mL of tetrahydrofuran into the three-necked flask, add 5.4g of sodium borohydride (0.1426mol) powder under stirring, continue stirring at 60°C for 15min, add dropwise 30mL of methanol, and reflux React until TLC (chloroform:methanol=4:1) monitors the completion of the reaction, concentrate in vacuum while hot, distill 40mL of the mixed solution, add 1gNaOH, hydrolyze at 70-80°C for 7-8h, separate the organic phase, anhydrous sulfuric acid Sodium-dried and concentrated to obtain 1.6 g of light yellow solid powder of 2-amino-3-pyridinemethanol (Intermediate A), m.p.67.5-68°C, yield 70%.
[0041]
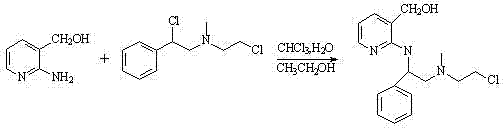
[0042] Get 7.3g N-(2-chloroethyl)-N-methyl-α-chloro-β-phenethylamine (0.0314mol) and join in the mixed solution of 7mL chloroform and 3mL water, 3g Intermediate A (0.0242 mol) was dissolved in 18mL chloroform with 0.075mL ethanol, slowly added dropwise to the above mixed solution, reacted for 5-6h, and solid K was added to th...
Embodiment 2
[0048] Add 5g of ethyl 2-aminonicotinate (0.0302mol) and 80mL of tetrahydrofuran into the three-neck flask, add 13g of sodium borohydride (0.2416mol) powder under stirring, continue stirring at 65°C for 15min, add dropwise 65mL of methanol, and react under reflux To TLC (chloroform: methanol = 4: 1) to monitor the completion of the reaction, concentrated in vacuum while hot, distilled 40mL of the mixed solution, added 1gNaOH, hydrolyzed at 70 ~ 80 ° C for 7 ~ 8h, separated the organic phase, anhydrous sodium sulfate Drying and concentration gave 2.7 g of 2-amino-3-pyridinemethanol (Intermediate A) light yellow solid powder, m.p.67.5-68°C, yield 75%.
[0049] Get 10g of N-(2-chloroethyl)-N-methyl-α-chloro-β-phenylethylamine (0.0430mol) into a mixed solution of 15mL of dichloromethane and 4mL of water, 4g of Intermediate A ( 0.0323mol) was dissolved in 25mL dichloromethane with 0.1mL ethanol, slowly added dropwise to the above mixed solution, reacted for 5-6h, and solid K was ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com