A kind of L-carnitine-producing Escherichia coli genetically engineered bacteria and its construction method and application
A technology of genetically engineered bacteria and Escherichia coli, applied in the field of bioengineering, can solve the problems of high production cost, intolerance to croton betaine, and failure to reach the level of industrial production, and achieve the effect of simple control and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1. Construction of Escherichia coli genetic engineering bacteria producing L-carnitine
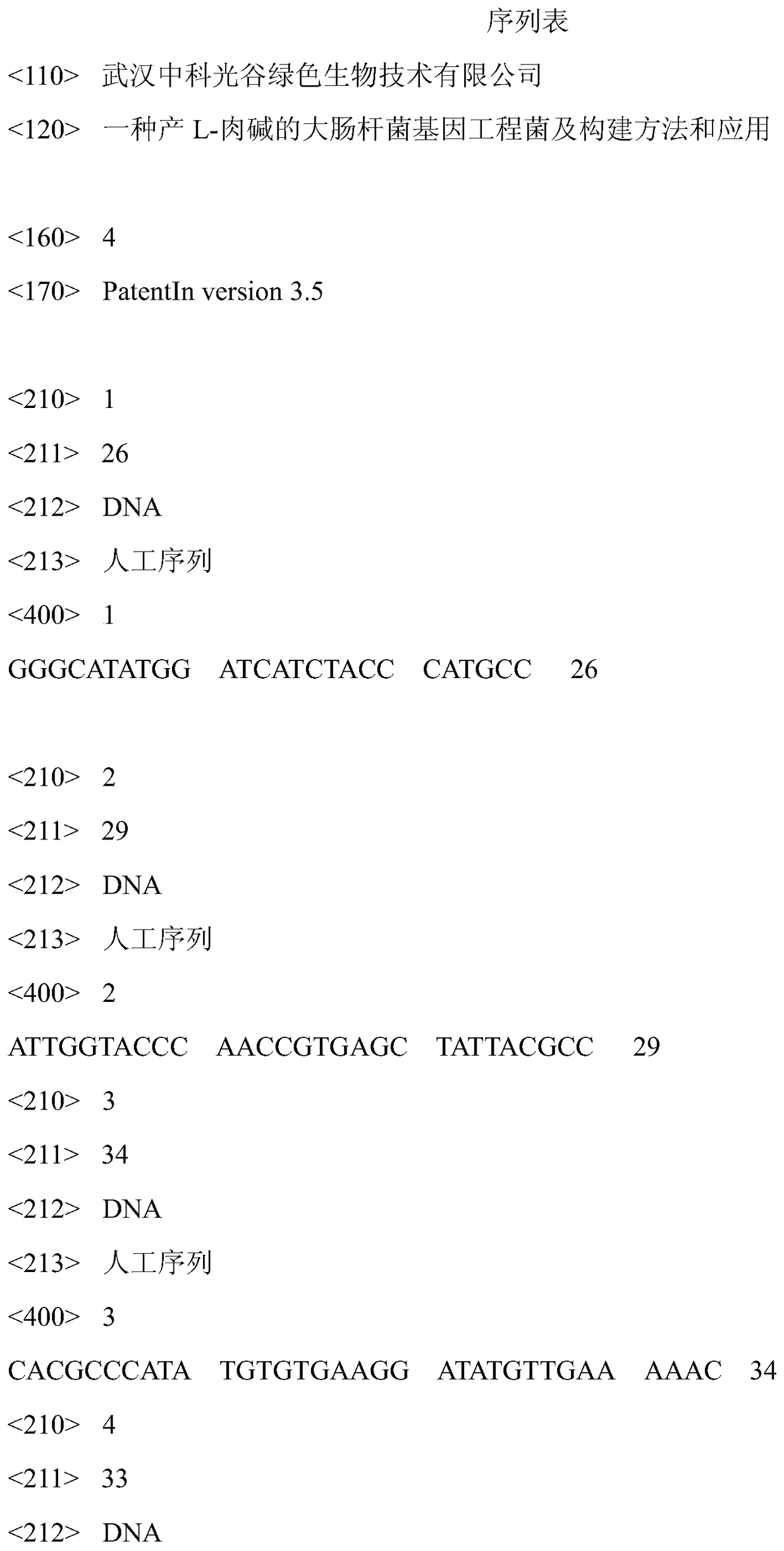
[0033] (1) According to the caiTABCDE operon in the genome sequence of Escherichia coli, design forward primer F-caiBCD-NdeI:5'-GGGCATATGGATCATCTACCCATGCC-3 (SEQ ID NO.1)' and reverse primer R-caiBCD-KpnI:5 '-ATTGGTACCCAACCGTGAGCTATTACGCC-3' (SEQ ID NO.2) or R-caiBCD-XhoI:5'-ATTCTCGAGCAACCGTGAGCTATTACGCC-3' (SEQ ID NO.9), NdeI and KpnI restriction sites (underlined) were added to both ends of the primer.
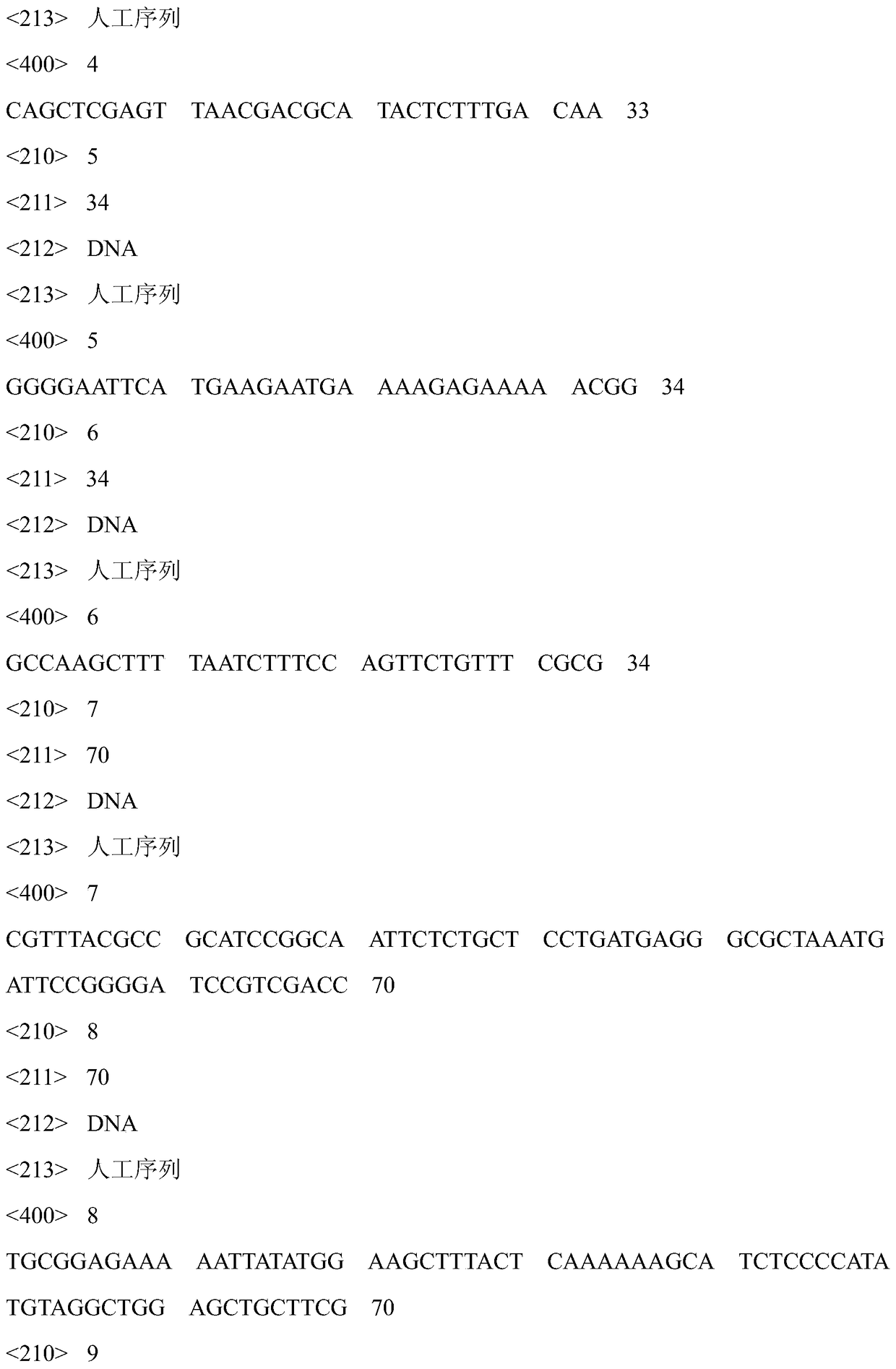
[0034] Forward primer F-caiF-NdeI:5'-CACGCCCAT ATGTGTGAAGGATATGTTGAAAAAC-3'(SEQ ID NO.3) and reverse primer R-caiF-xhoI:5'-CAGCTCGAGTTAACGACGCATACTCTT TGACAA-3'(SEQ ID NO.4) of CaiF ). Forward primer F-caiT-EcoRI of caiT: 5'-GGGGAATTCATGAAGAATGAAAAGAGAAAAACGG-3' (SEQ ID NO.5), reverse primer R-caiT-HindIII: 5'-GCCAAGCTTTTAATCTTTCCAGTTCTGTTTCGCG-3' (SEQ ID NO.6).
[0035] (2) Using Escherichia coli genomic DNA as a template, using TaKaRa’s DNA polymerase primestar and designed pr...
Embodiment 2
[0052] 1. Construction of Escherichia coli genetic engineering bacteria producing L-carnitine
[0053] Step 1: Amplify the caiBCD fragment from the genome of Escherichia coli wild strain BW25113, the primer design is forward: 5'-GGGCATATGGATCATCTACCCATGCC-3' (SEQ ID NO.1); the primer design is reverse: 5'-ATTCTCGAGCAACCGTGAGCTATTACGCC-3 '(SEQ ID NO.9); Step 2: select the plasmid pET28a of an IPTG-induced promoter as a carrier, insert the caiBCD fragment at the NdeI / XhoI restriction site of the multi-cloning site, and obtain the overexpression plasmid pET28a-caiBCD; step Three: Transfer the overexpression plasmid pET-caiBCD into the E. coli expression strain BL21(DE3) to obtain the genetically engineered bacteria BL21(DE3) / pET28a-caiBCD; Step four: Extend the genes caiF and caiT, caiF from E. coli W3110 The forward and reverse primers were: 5'-CACGCCCAT ATGTGTGAAGGATATGTTGAAAAAC-3'(SEQ NO.3) and 5'-CAGCTCGAGTTAACGACGCATACTCTTTGACAA-3'(SEQ NO.4). The forward and reverse primers...
Embodiment 3
[0063] 1. Construction of Escherichia coli genetic engineering bacteria producing L-carnitine
[0064] Same as step 1 of Example 2 above.
[0065] 2. Preparation of the whole cell enzyme source of Escherichia coli genetically engineered bacteria BL21(DE3) / (pET28a-caiBCD+pACYCD-caiT-caiF)
[0066] (1) Preparation of enzyme production medium: glycerol 10ml / L, peptone 5g / L, yeast powder 15g / L, KH 2 PO 4 5g / L, K 2 HPO 4 5g / L, sodium fumarate 4g / L and croton betaine 4g / L, pH 6. Sterilize.
[0067] (2) The inoculum size is 1% (V / V). After the Escherichia coli genetically engineered bacteria are gradually activated and cultured, they are placed in a container equipped with an enzyme-producing medium, and 1 mM IPTG is added for aerobic culture at 37° C. for 6 hours.
[0068] (3) Centrifuge at 10° C., 8000 rpm, and collect the bacterial cells. The cells were washed twice with 67 mmol / l phosphate buffer at pH 7.4.
[0069] 3. Whole-cell enzymatic conversion of croton betaine to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com