A novel acyl lactone derivative and its preparation method
A technology of derivatives and acyl lactones, which is applied in the fields of chemical synthesis and pharmaceuticals, and can solve problems such as unsatisfactory anti-drug-resistant bacteria activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
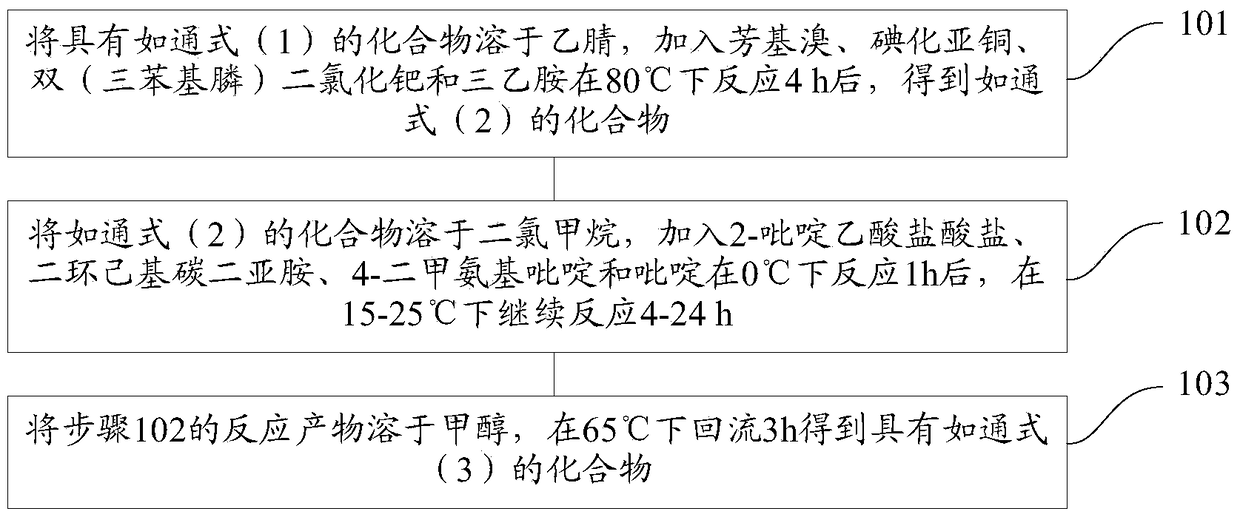
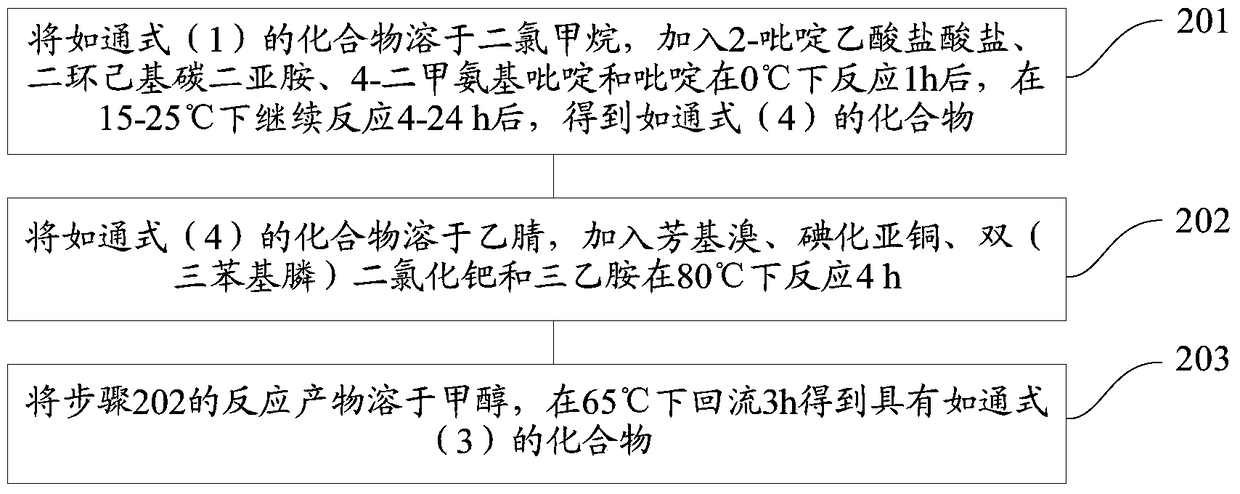
[0101] The present invention also provides a preparation method of novel acyl lactone derivatives, the specific steps comprising:
[0102] Step 1. Dissolve the compound of general formula (1) in acetonitrile, add aryl bromide, cuprous iodide, bis(triphenylphosphine)palladium dichloride and triethylamine to react at 80°C for 4h, Obtain as the compound of general formula (2);
[0103] Step 2. Dissolve the compound of general formula (2) in dichloromethane, add 2-pyridineacetic acid hydrochloride, dicyclohexylcarbodiimide, 4-dimethylaminopyridine and pyridine and react at 0°C for 1h After that, continue to react at 15-25°C for 4-24h;
[0104] Step 3, dissolving the reaction product of step 2 in methanol, and refluxing at 65° C. for 3 h to obtain a compound having general formula (3);
[0105]
[0106] In the embodiment of the present invention, preferably, in the general formula (2) and the general formula (3),
[0107] The groups represented by R in the general formula (2)...
example 1
[0156] First preparation: 2'-O-acetyl-3-O-descladinose-3-hydroxy-6-O-methylerythromycin A9-O-[3-(3'-(6'-nitro )pyridyl)-2-propynyl]oxime-11,12-cyclocarbonate (2a)
[0157] Dissolve 1.00 g (1.41 mmol) of compound 1 in acetonitrile (10 mL), add bis(triphenylphosphine) palladium dichloride (51 mg, 0.073 mmol), cuprous iodide (27 mg, 0.014 mmol), triethylamine (0.30 mL, 2.11 mmol), 2-nitro-5-bromopyridine (865 mg, 4.26 mmol). The reaction system was replaced with argon gas 8 times, and stirred in an oil bath at 80° C. for 4 h under the protection of argon gas. After the reaction was completed, distilled water (10 mL) was added to terminate the reaction, extracted twice with ethyl acetate (20 mL), the upper organic phase was washed with saturated brine (10 mL), and distilled under reduced pressure to obtain a dry solid. Compound 2a (248 mg, 0.30 mmol, 21.3%) was obtained by column chromatography (100-200 mesh silica gel, mobile phase V (dichloromethane): V (ethanol): V (ammonia) ...
example 2
[0166] First preparation: 2'-O-acetyl-3-O-descladinose-3-hydroxy-6-O-methylerythromycin A9-O-[3-(2'-(6'-amino) Pyridyl)-2-propynyl]oxime-11,12-cyclocarbonate (2b)
[0167] Dissolve 1.00 g (1.41 mmol) of compound 1 in acetonitrile (10 mL), add bis(triphenylphosphine) palladium dichloride (51 mg, 0.073 mmol), cuprous iodide (27 mg, 0.014 mmol), triethylamine (0.30 mL, 2.11 mmol), 2-amino-6-bromopyridine (731 mg, 4.22 mmol). The reaction system was replaced with argon gas 8 times, and stirred in an oil bath at 80° C. for 4 h under the protection of argon gas. After the reaction was completed, distilled water (10 mL) was added to terminate the reaction, extracted twice with ethyl acetate (20 mL), the upper organic phase was washed with saturated brine (10 mL), and distilled under reduced pressure to obtain a dry solid. Compound 2b (291 mg, 0.36 mmol, 25.5%) was obtained by column chromatography (100-200 mesh silica gel, mobile phase V (dichloromethane): V (ethanol): V (ammonia) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com