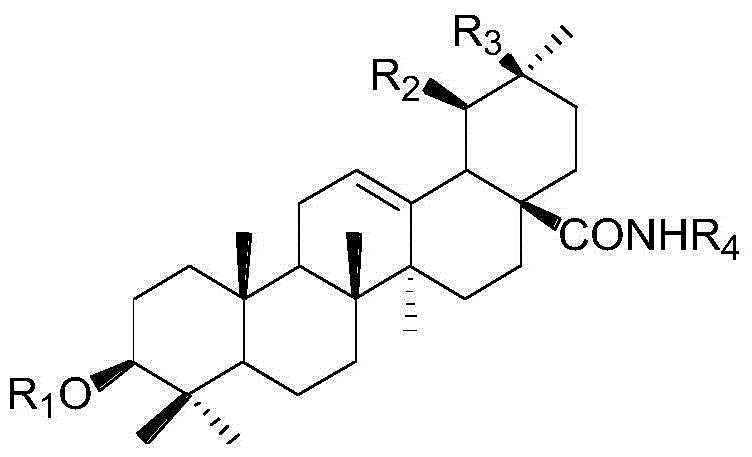
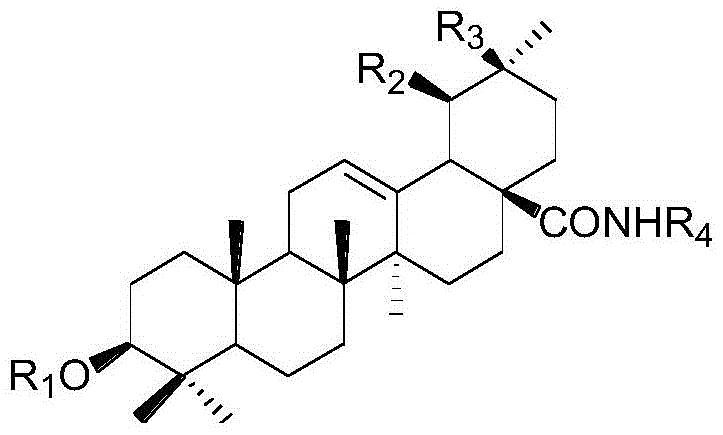
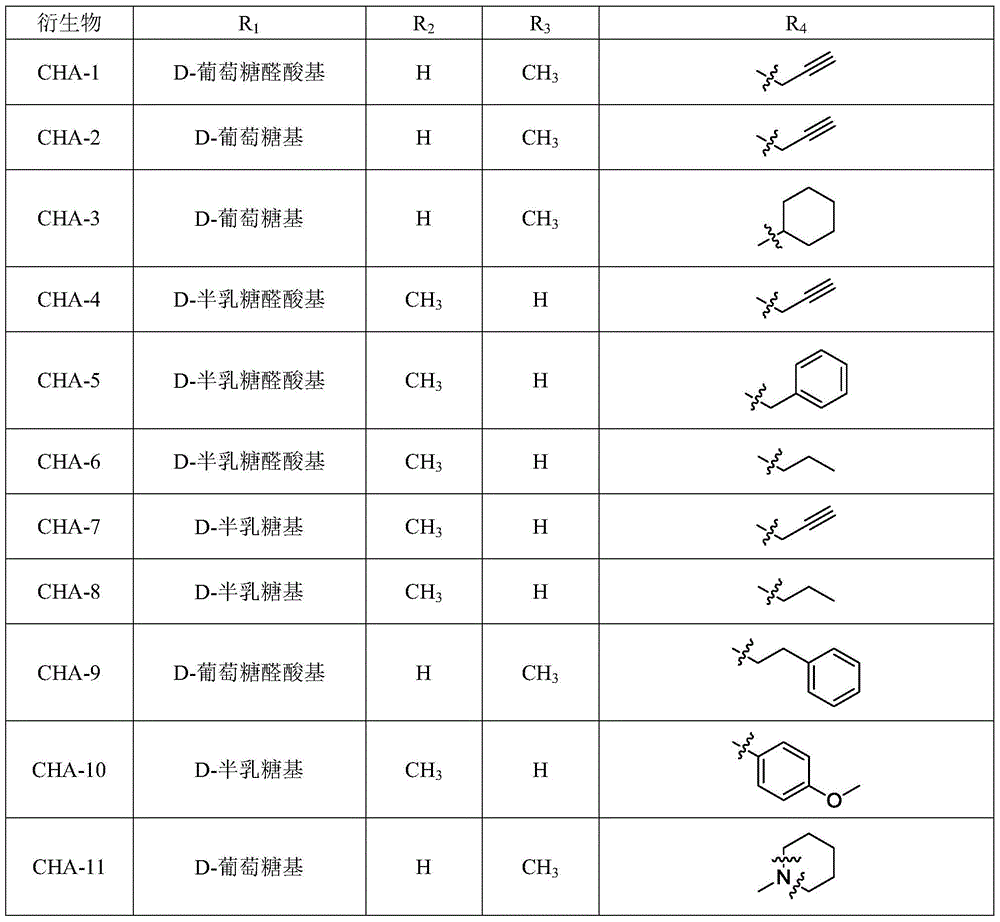
3‑Monouronic acid oxyglycoside oleanane type and ursane type triterpene saponin derivatives and preparation method and application thereof
A kind of uronic acid oxyglycoside oleanane and oleanane type technology, which is applied in the field of 3-monouronic acid oxyglycoside oleanane type and ursane type triterpene saponin derivatives and their preparation, It can solve the problems of low bioavailability and poor solubility, and achieve the effects of enhancing antiarrhythmic activity, good cardioprotective effect, and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Preparation of Derivatives CHA-1 and CHA-2
[0034]
[0035] Step a'-step d': Preparation of glucose trichloroacetimidate (5')
[0036] Under an ice-water bath, dissolve D-glucose (5.0 g, 27.7 mmol) in 100 mL of dry pyridine, add benzoyl chloride (19.3 mL, 166.5 mmol), stir and react at room temperature for 18 h, add 60 mL of water to destroy excess benzoyl chloride, and Extract with ethyl acetate, combine the organic phases, wash with water, dilute hydrochloric acid, and saturated sodium bicarbonate in sequence, dry over anhydrous magnesium sulfate, filter, and concentrate the filtrate to obtain 18.7 g of perbenzoylated glucose (2').
[0037] Dissolve 4.0 g of perbenzoylated glucose (2') obtained in the previous step in 40 mL of dry dichloromethane, add 12 mL of HBr-HOAc dropwise, and stir the reaction at room temperature. Washed with sodium solution and brine, and the organic layer was concentrated to dryness to obtain the glucose-terminal brominated pro...
Embodiment 2
[0053] Embodiment 2 prepares derivative CHA-3
[0054]
[0055] The product oleanolic acid-3-O-(2,3,4,6-tetra-O-benzoyl-β-D-glucopyranoside) (O-3)( 500mg, 0.488mmol) was dissolved in 5mL of dry dichloromethane, EDCI (140mg, 0.732mmol) and HOBT (98.8mg, 0.732mmol) were added and stirred at room temperature for 2h, and cyclohexylamine (1.64mmol ), the reaction was continued for 4 h at room temperature. After the completion of the reaction was monitored by TLC, it was washed with 0.1N dilute hydrochloric acid, saturated aqueous sodium bicarbonate, and saturated aqueous sodium chloride in sequence, and dried overnight with anhydrous sodium sulfate. Suction filtration, evaporated to dryness under reduced pressure, then dissolved in 5mL dry methanol and dichloromethane (v / v=3:1), added 0.8mL sodium methoxide methanol (1mol / L) solution, reacted at room temperature for 6h. After the reaction, use 732-type cation exchange resin to adjust the pH to neutral, filter and evaporate to ...
Embodiment 3
[0057] Embodiment 3 prepares derivative CHA-5
[0058]
[0059]1) The preparation of galactose trichloroacetimide ester is similar to the preparation method of glucose trichloroacetimide ester in Example 1. Under an ice-water bath, D-galactose (5.0 g, 27.7 mmol) was dissolved in 100 mL of dry pyridine , add benzoyl chloride (19.3mL, 166.5mmol), stir at room temperature for 18h, add 60mL of water to destroy excess benzoyl chloride, extract with ethyl acetate, combine organic phases, wash with water, dilute hydrochloric acid, saturated sodium bicarbonate in sequence , dried over anhydrous magnesium sulfate, filtered, and the filtrate was concentrated to obtain 15.9 g of perbenzoylated galactose. Dissolve 4.0 g of perbenzoylated galactose obtained in the previous step in 40 mL of dry dichloromethane, add 12 mL of HBr-HOAc dropwise, and stir the reaction at room temperature. , brine, and the organic layer was concentrated to dryness to obtain a terminal brominated product of g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com