Application of Schisandra C in the preparation of drugs for improving glucocorticoid-induced osteoporosis
A technology of schisandra C and glucocorticoids, applied in the field of medicine, can solve the problems of less research, achieve the effect of improving osteoporosis, improving glucocorticoid-induced osteoporosis, and promoting osteoblast proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
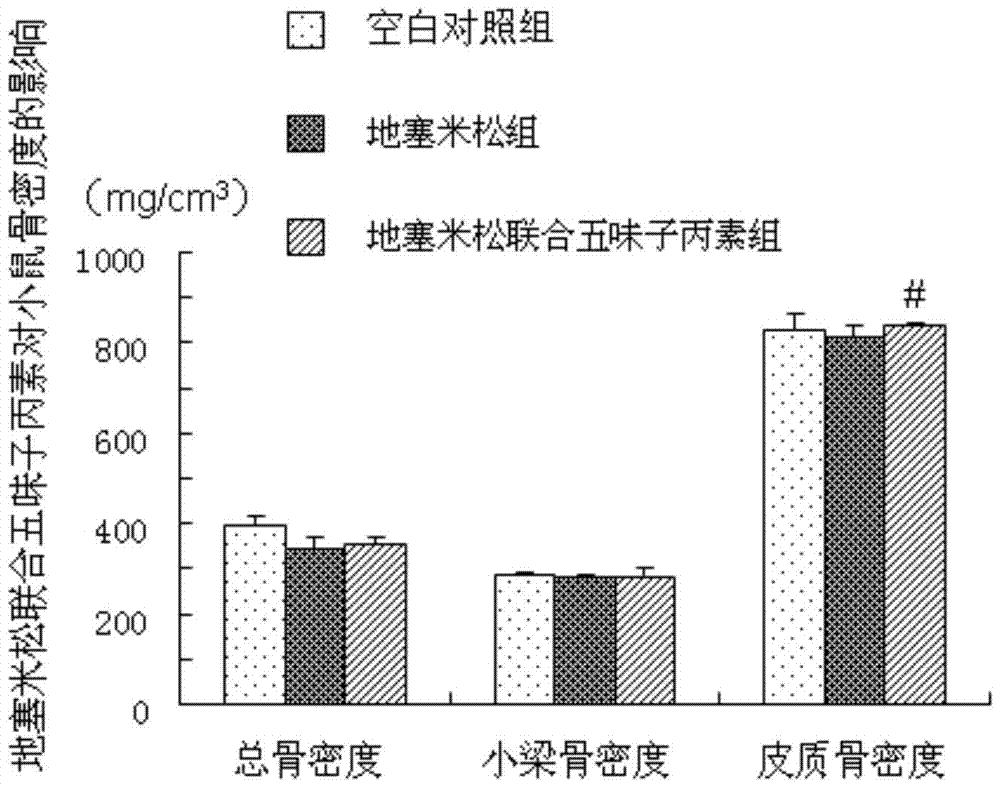
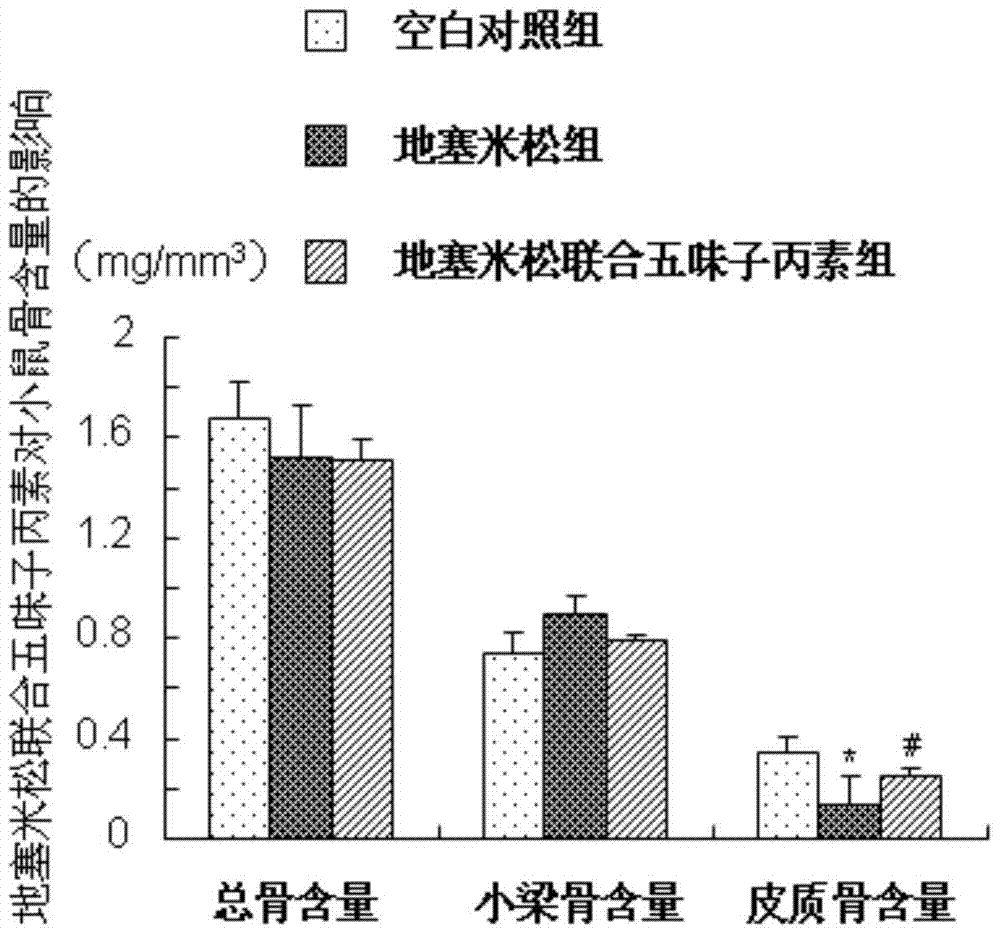
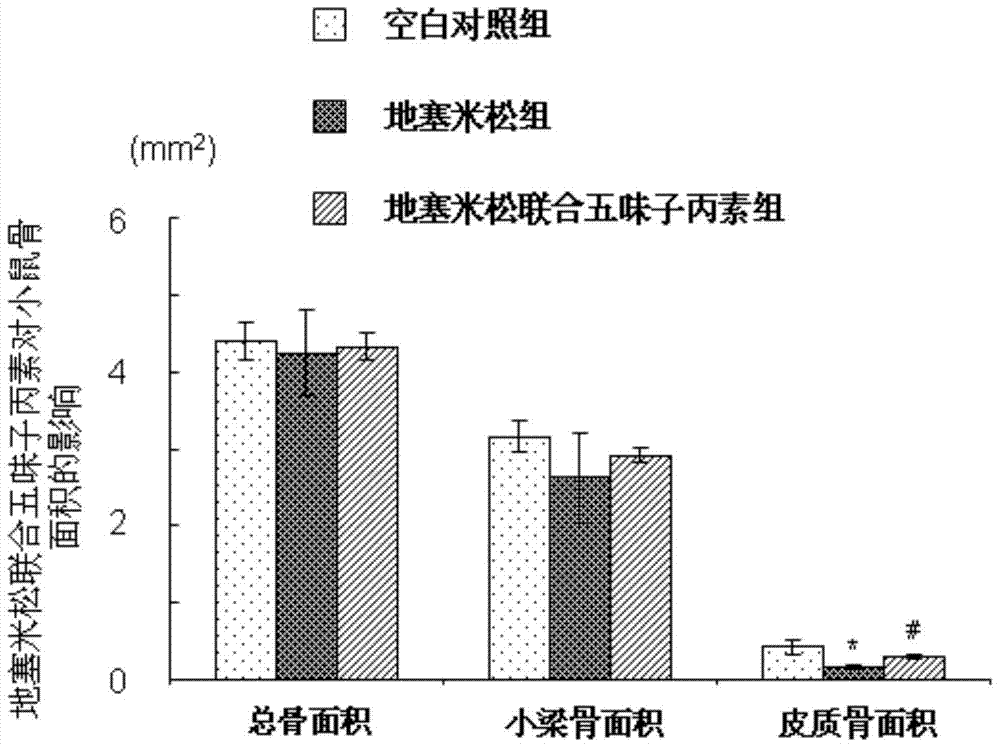
[0029] Example 1 (Schisandra C improves the study of dexamethasone-induced osteoporosis in mice)
[0030] (1) Materials and methods
[0031] (1) Experimental animals
[0032] 40 adult male ICR mice (8 weeks old, with an average body weight of 30±2g) were provided by the Experimental Animal Center of Second Military Medical University. Raised in the SPF animal experiment center, 12 hours a day, day and night, constant temperature 18 ~ 20 ℃, mice can eat and drink freely. All mice were reared for one week in advance, and the experiment was carried out after adapting to the living environment.
[0033] (2) Preparation of main reagents
[0034] 1. Dexamethasone sodium phosphate: use 0.9% sodium chloride solution to prepare a solution with a concentration of 0.25mg / ml;
[0035] 2. Schisandra C: Dissolve 20mg of Schisandra C in 2100ml of absolute ethanol, pipette until completely dissolved; dissolve 20mg of completely dissolved Schisandra C in 84ml of propylene glycol, the final...
Embodiment 2
[0048] Example 2 (the effect of Schisandra C combined with dexamethasone on the proliferation and differentiation of mouse osteoblasts)
[0049] (1) Materials and methods
[0050] Cell line: mouse osteoblast cell line (MC3T3-E1) was purchased from ATCC. The above cell lines were incubated in α-MEM medium containing 10% fetal bovine serum at 37°C with a volume fraction of 5% CO 2 , Routine culture under fully saturated humidity conditions, replace the medium for 48 hours, when the cell growth reaches saturation, digest with 0.25% trypsin + 0.02% EDTA for passage, once every 2-3 days, the experiment uses logarithmic growth phase cells.
[0051] 1. The effect of Schisandra C on the proliferation inhibition of mouse osteoblasts induced by dexamethasone
[0052] 1.1. The osteoblasts MC3T3-E1 in the logarithmic growth phase were inoculated in three 96-well plates, and the cell density was adjusted to about 5×10 3 Each culture plate is divided into 7 groups, namely ① blank control...
Embodiment 3
[0071] Embodiment 3 (preparation of schisandra acetin tablet)
[0072] Take 25g of schisandra acetone, 10g of hydroxypropyl methylcellulose, 50g of microcrystalline cellulose, 5g of croscarmellose sodium, mix well, add an appropriate amount of 60% ethanol to make a soft material, pass through a 24-mesh sieve and granulate , dried at 50°C for 2 hours, the dried granules were passed through a 30-mesh sieve for granulation, 2.5g of magnesium stearate was added, mixed evenly, and pressed into 1000 tablets respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com