Epinephelus malabaricus Piscidin transformation body antimicrobial peptide and application thereof
A Malabar grouper and antimicrobial peptide technology, applied in the biological field, can solve the problems of large-scale production, application difficulties, change of cell permeability, destruction, etc., achieve good application prospects, reduce hemolytic activity and cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
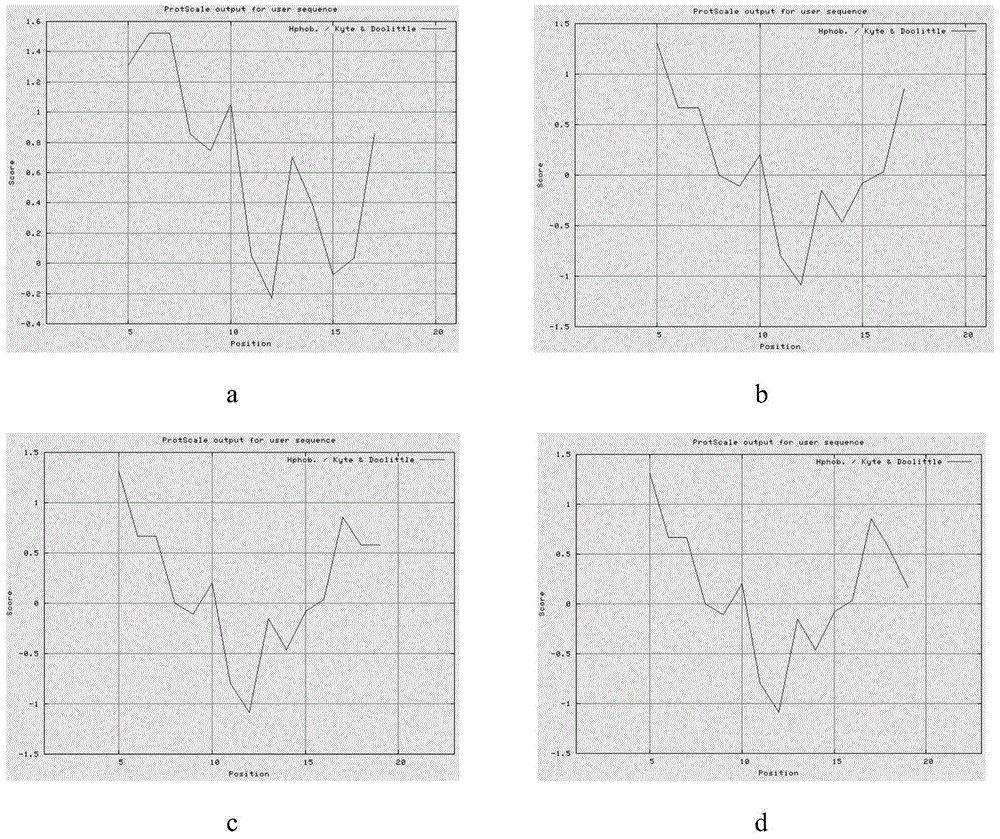
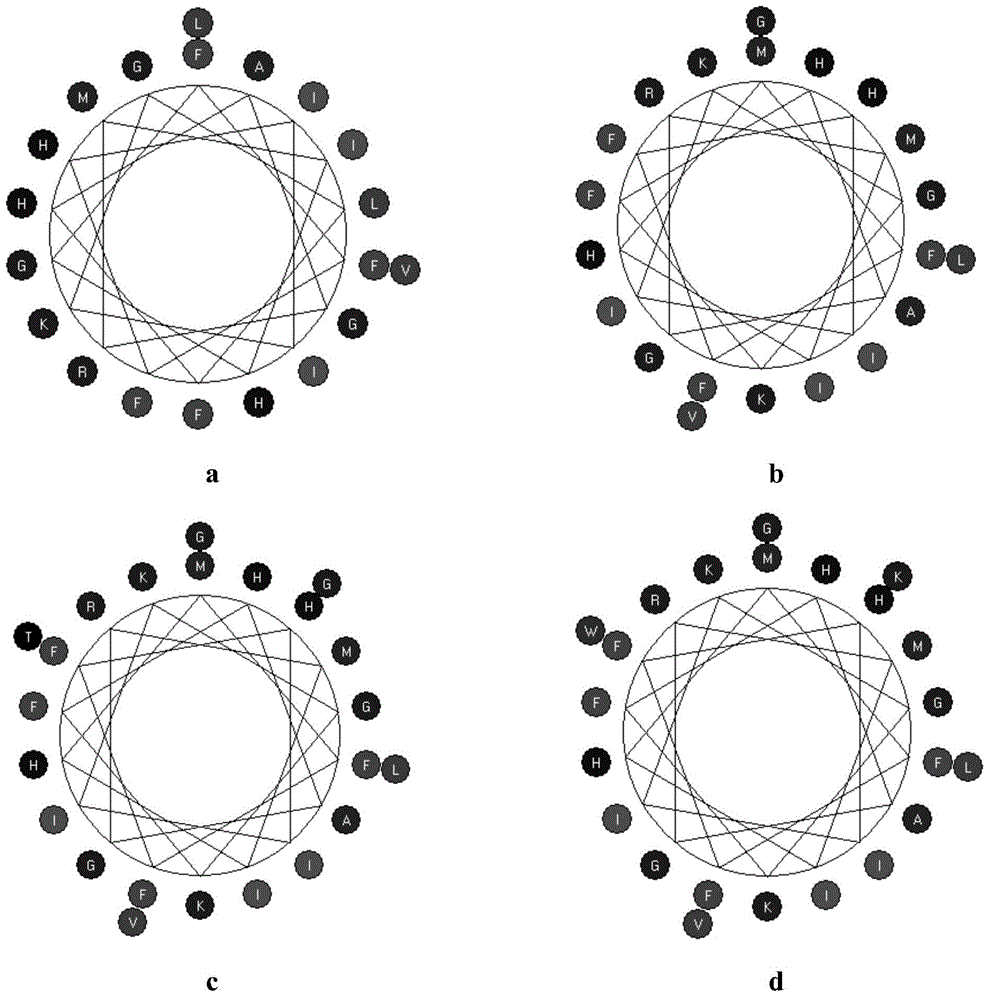
[0066] [Example 1]: Prediction and analysis of polypeptide sequence structure
[0067]Select the Kyte&Doolittle model on the website http: / / web.expasy.org / cgi-bin / protscale / protscale.pl to analyze the hydrophobicity of the polypeptide, and predict it on the website http: / / www.expasy.org / tools / pi_tool.html The isoelectric point of the mature peptide predicted by its isoelectric point pI / Mw value was analyzed by bioedit software on the helcalwheeldiagram, and the software chemoffice2008 was used to predict the secondary structure of the above four peptides. Based on the amino acid sequence of emPis-wt, the polypeptide was transformed by replacing and adding amino acid residues, etc., without destroying the α-helical structure, appropriately weakening the hydrophobicity of the polypeptide and increasing the isoelectric point, and finally determined three modified peptides for use In subsequent studies, they were named pisL9K20, pisL9K22TG and pisL9K22WK, respectively.
[0068] D...
Embodiment 2
[0069] [Example 2]: peptide synthesis
[0070] The designed antimicrobial peptides were synthesized by solid-phase chemical synthesis, purified by reverse-phase high-performance liquid chromatography (purity>95%), and identified by electrospray mass spectrometry. This procedure was performed by Shanghai Sangon Biotech Co., Ltd. (Shanghai).
Embodiment 3
[0071] [Example 3]: antibacterial experiment
[0072] 3.1LB medium configuration
[0073] LB medium formula: 10 g of bacto-tyrptone for bacterial culture, 5 g of bacto-yeastextract for bacterial culture, and 10 g of NaCl. After dissolving in deionized water, add 5 mol / L NaOH (about 0.2 mL) dropwise to adjust the pH value to 7.0, add deionized water to adjust the medium volume to 1 L, and sterilize at high temperature and high pressure for later use. Add 1.5% (m / v) agar powder to the solid medium.
[0074] 3.2 Bacterial culture: Take 20 μL of preserved strains of Escherichia coli (Escherichiacoli) TOP10 and Staphylococcus aureus (Staphylococcus aureus) and inoculate them into 20 mL of liquid medium respectively, and culture them overnight at 37°C. Take 200 μL of overnight culture and transfer it to 20 mL of sterile medium, culture at 37°C and 200 rpm for 1-2 hours, so that the OD600 is about 0.2, at this time, the bacterial cell concentration reaches 5×107 cfu / mL, which is th...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap