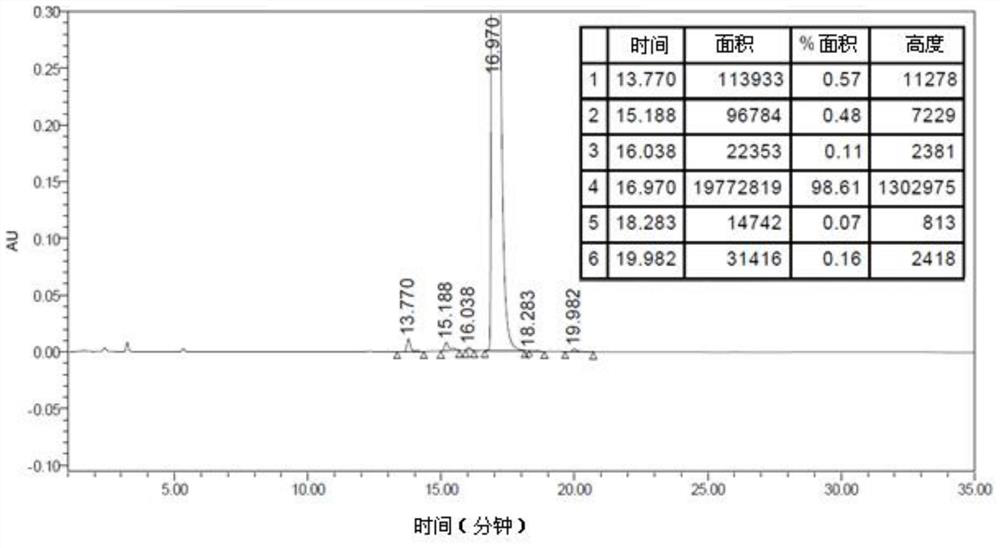
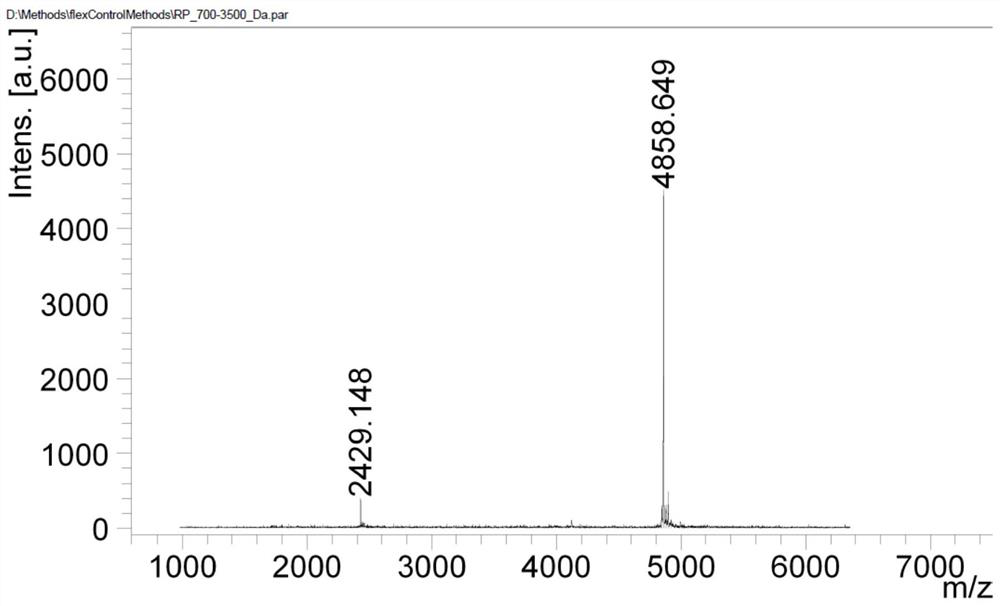
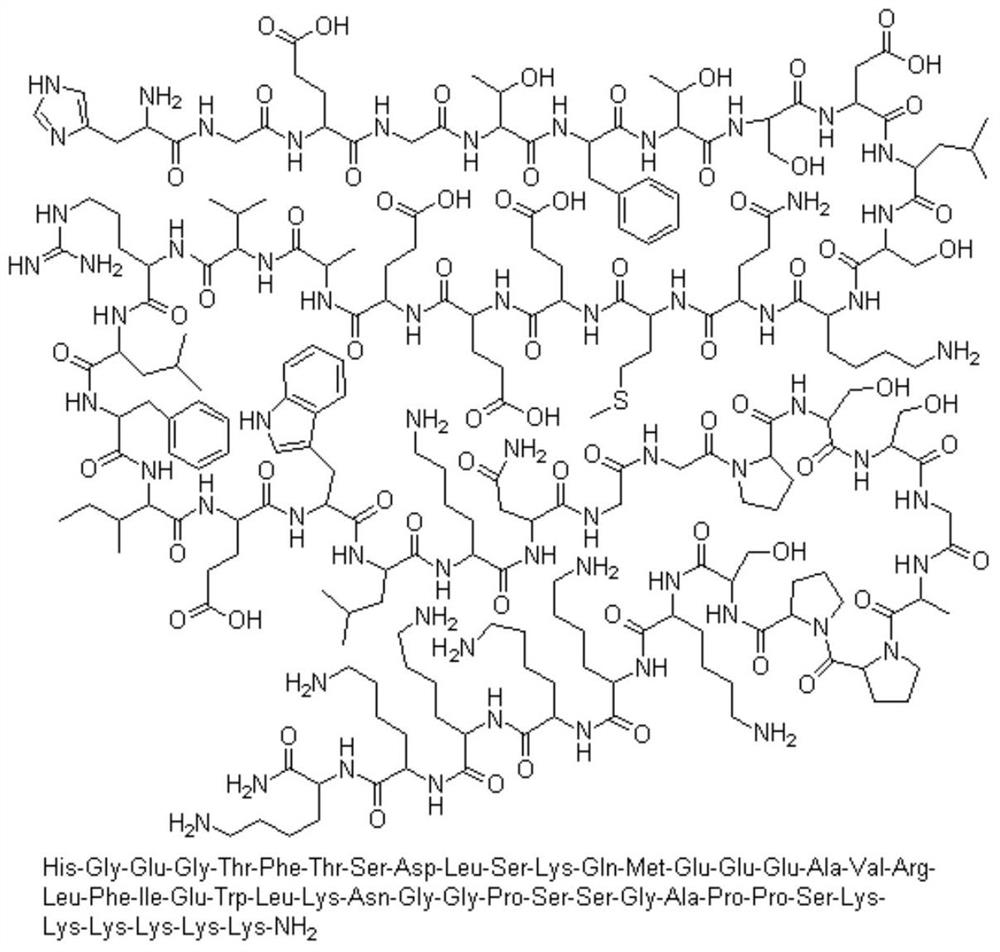
A kind of method for preparing lixisenatide
A lixisenatide and resin technology, applied in the field of medicine, can solve problems such as increasing the difficulty of purification, reducing the purity of crude peptides, and reducing product yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] H-Arg(pbf)-Leu-Phe-Ile-Glu(OtBu)-Trp(Boc)-Leu-Lys(Boc)-Asn(Trt)-Gly-Gly-Pro-Ser(tBu)-Ser(tBu) -Synthesis of Gly-Ala-Pro-Pro-Ser(tBu)-Lys(Boc)-Lys(Boc)-Lys(Boc)-Lys(Boc)-Lys(Boc)-Lys(Boc)-Rink Amide Resin
[0093] Weigh 100g of Rink Amide Resin (purchased from Tianjin Nankai Hecheng) with a substitution degree of 0.1mmol / g and add it to the solid-phase reaction column, wash it twice with DMF of one volume of resin bed, and wash it twice with one volume of resin bed Volumetric DMF swells the resin for 30 minutes. Add one volume of resin bed layer 20% piperidine / DMF, remove Fmoc by 10min+10min. The reaction solution was drained, and the resin was washed 6 times with DMF of one volume of the resin bed. Weigh 14.055g Fmoc-Lys(Boc)-OH (30mmol), 4.053g HOBt (30mmol) and dissolve in the minimum volume mixed solution of DCM and DMF with a volume ratio of 1:1, add 5.19ml DIC (33mmol) under ice-water bath to activate After 3 minutes, it was added to the solid-phase reaction col...
Embodiment 2
[0095] H-Arg(pbf)-Leu-Phe-Ile-Glu(OtBu)-Trp(Boc)-Leu-Lys(Boc)-Asn(Trt)-Gly-Gly-Pro-Ser(tBu)-Ser(tBu) -Synthesis of Gly-Ala-Pro-Pro-Ser(tBu)-Lys(Boc)-Lys(Boc)-Lys(Boc)-Lys(Boc)-Lys(Boc)-Lys(Boc)--Rink Amide Resin
[0096]Weigh 25g of Rink Amide Resin (purchased from Tianjin Nankai Hecheng) with a substitution degree of 0.4mmol / g, add it to the solid-phase reaction column, wash twice with DMF of double the volume of resin bed, and wash twice with double volume of resin bed Layer volume DMF swells the resin for 30 minutes. Add 20% piperidine / DMF to double the volume of the resin bed, and remove Fmoc in the manner of 10min+10min. The reaction solution was drained, and the resin was washed 6 times with DMF of one volume of the resin bed. Weigh 14.055g Fmoc-Lys(Boc)-OH (30mmol), 4.053g HOBt (30mmol) and dissolve in the minimum volume mixed solution of DCM and DMF with a volume ratio of 1:1, add 5.19ml DIC (33mmol) under ice-water bath to activate After 3 minutes, it was added to ...
Embodiment 3
[0098] H-Arg(pbf)-Leu-Phe-Ile-Glu(OtBu)-Trp(Boc)-Leu-Lys(Boc)-Asn(Trt)-Gly-Gly-Pro-Ser(tBu)-Ser(tBu) -Synthesis of Gly-Ala-Pro-Pro-Ser(tBu)-Lys(Boc)-Lys(Boc)-Lys(Boc)-Lys(Boc)-Lys(Boc)-Lys(Boc)-Rink Amide Resin
[0099] Weigh 50g of Rink Amide Resin (Tianjin Nankai Hecheng) with a substitution degree of 0.2mmol / g, add it to the solid-phase reaction column, wash it twice with DMF of double the resin bed volume, and wash twice with double the resin bed volume DMF swells the resin for 30 minutes. Add one volume of resin bed layer 20% piperidine / DMF, remove Fmoc by 10min+10min. The reaction solution was drained and the resin was washed 6 times with DMF. Weigh 14.055g Fmoc-Lys(Boc)-OH (30mmol), 4.053g HOBt (30mmol) and dissolve in the minimum volume mixed solution of DCM and DMF with a volume ratio of 1:1, add 5.19ml DIC (33mmol) under ice-water bath to activate After 3 minutes, it was added to the solid-phase reaction column and reacted at room temperature for 2 hours. Use the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com