Doxofylline lyophilized powder injection
A technology of freeze-dried powder injection and doxofylline, which is applied in the field of freeze-dried powder injection, can solve the problems of slowing down the sublimation speed of water, without coordinated control, and increasing energy consumption, so as to reduce the energy consumption of freeze-drying evaporation and reduce production cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
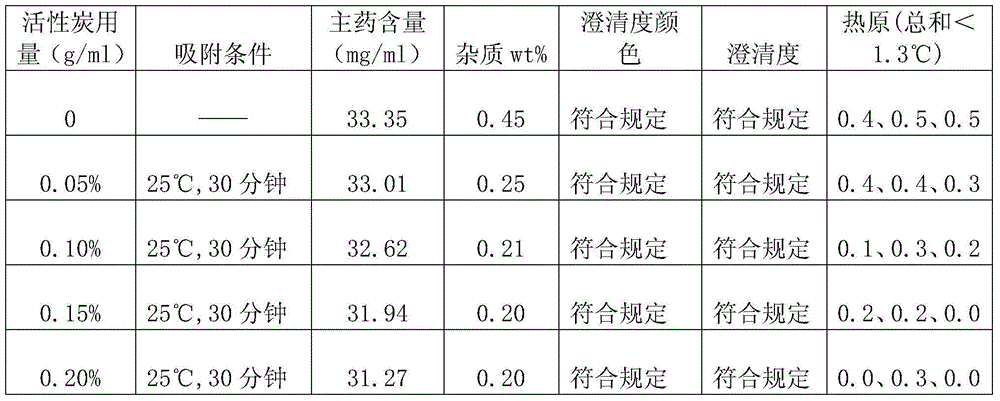
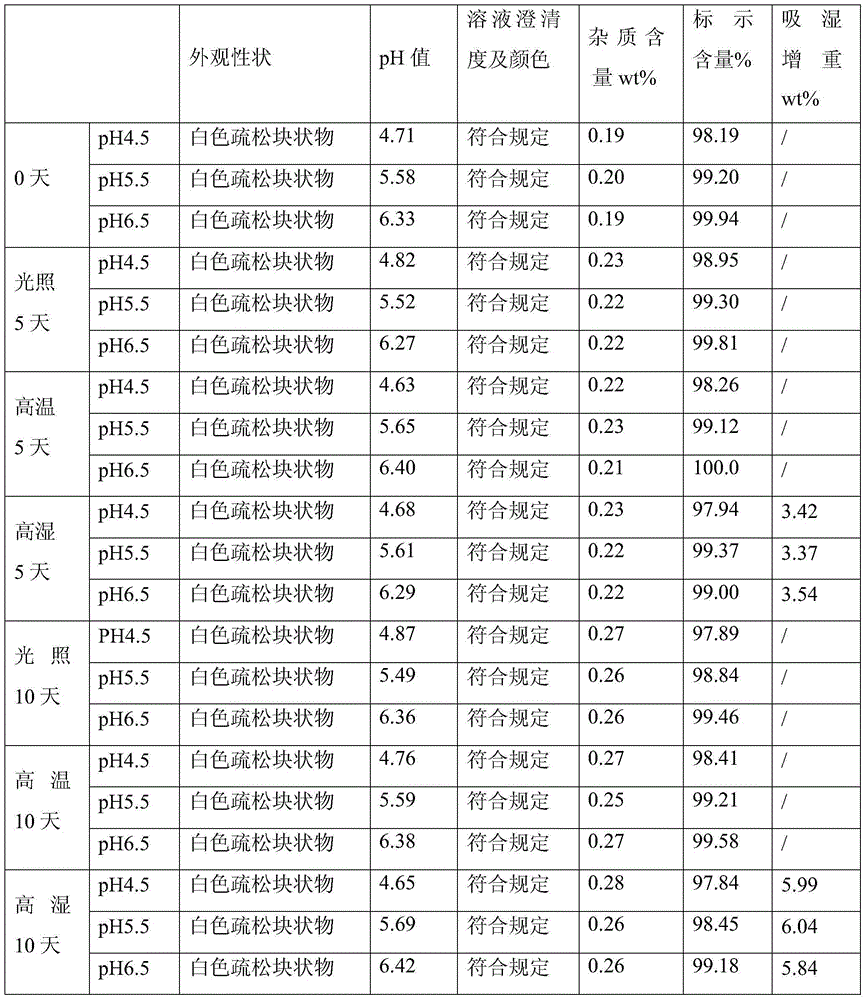
[0042] According to the following preparation method, add 200g doxofylline and 150g mannitol into a graduated container, add water for injection to 4800ml, stir while adding, add 6g of activated carbon, stir and absorb for 30 minutes, decarbonize, use 0.01 mol / L hydrochloric acid solution to adjust the pH value to 5.5, then add water for injection to 6000ml, sterilize and filter, and then fill it into a 20ml vial with a filling volume of 5.8-6.3ml / bottle, totaling 1000 bottles. Finally, the filled injection is put into a freeze dryer for freeze drying, and after the freeze drying is finished, it is plugged and capped to obtain the product.
[0043] Wherein, freeze-drying comprises following three steps:
[0044] (1) Pre-freezing: the pre-freezing temperature is controlled at -40°C, and the time is 1.5 to 3 hours;
[0045] (2) Sublimation: Vacuum control 10-30Pa, take 20 hours to raise the product temperature to 0°C;
[0046] (3) Analytical drying: take 8 hours to raise the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com