Gramine analogue with heterocyclic structure and application of gramine analogue with heterocyclic structure to preparation of anti-CVB3 virus medicines
A technology of analogs and heterocycles, which is applied in the application field of preparing anti-CVB3 virus drugs, can solve problems such as no antiviral activity found, and achieve the effects of improving application value and market competitiveness, being easy to purchase, and being inexpensive.
Inactive Publication Date: 2016-07-13
HUBEI UNIV OF TECH
View PDF0 Cites 9 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Gramine, as a natural indole alkaloid, has been isolated from various plant materials and coal tar, and exhibits a wide range of pharmaceutical activities, such as rela
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
 Login to View More
Login to View More PUM
 Login to View More
Login to View More Abstract
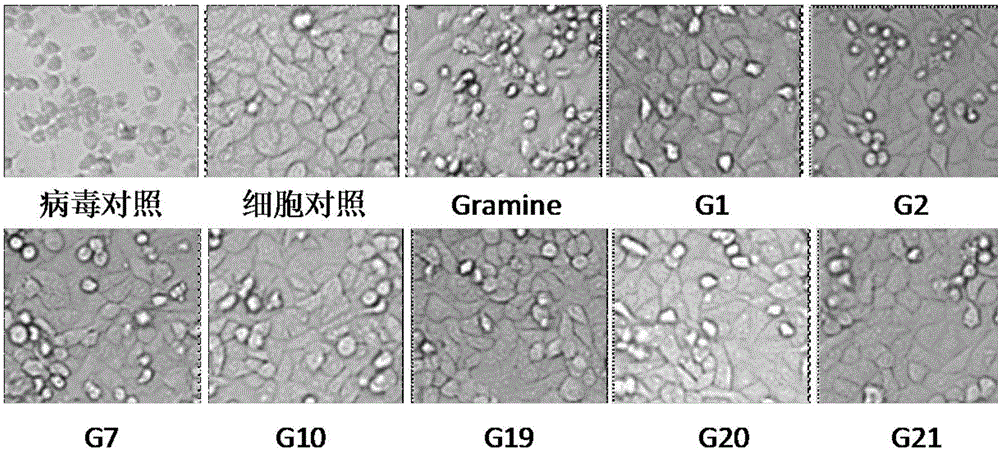
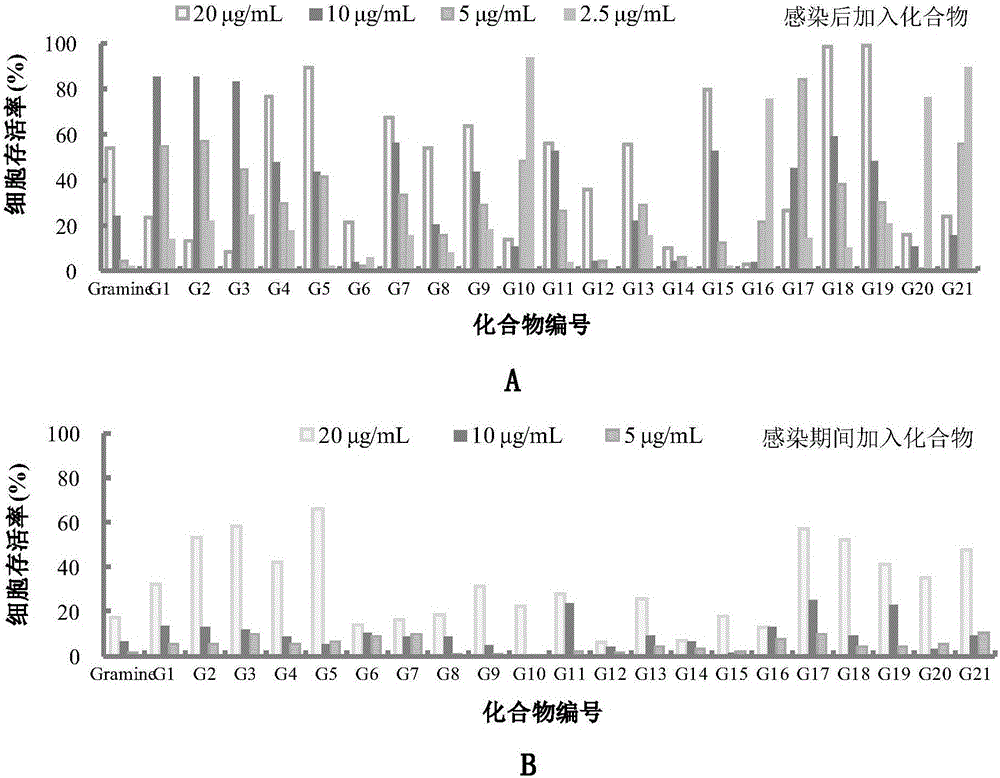
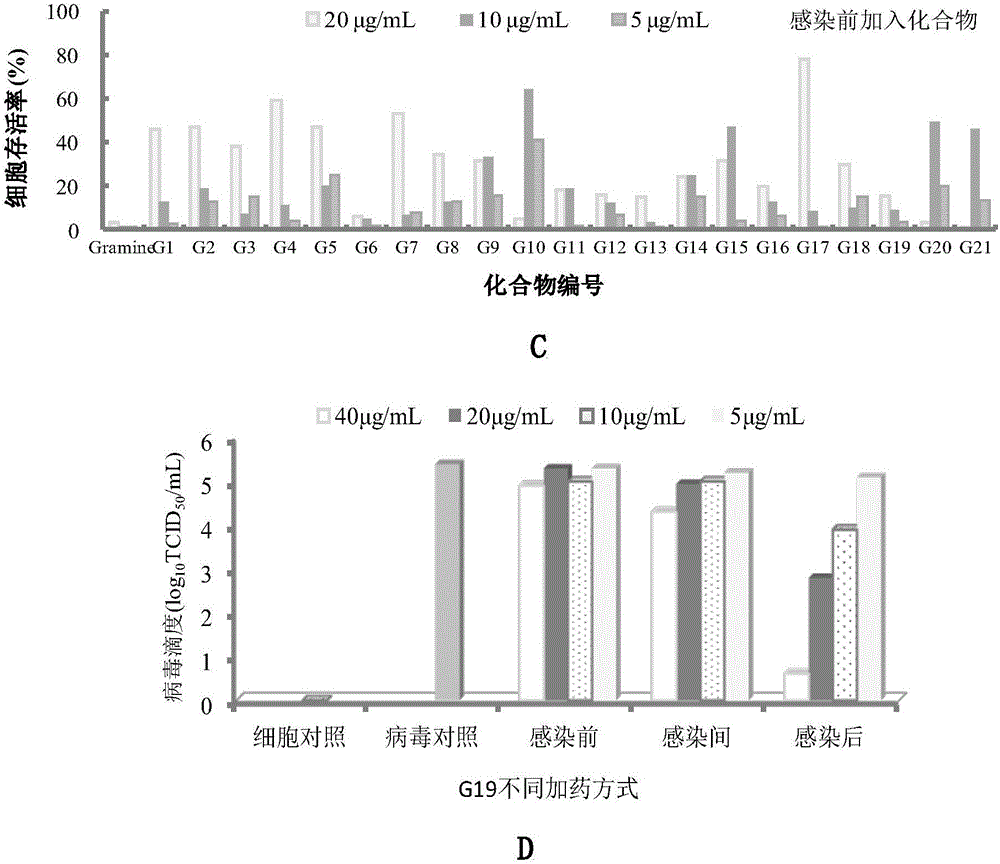
The invention relates to a gramine analogue with a heterocyclic structure and application of the gramine analogue with the heterocyclic structure to preparation of anti-CVB3 virus medicines.A chemical formula of the gramine analogue with the heterocyclic structure is as shown in the specification.According to anti-CVB3 activity research experiments of the gramine analogue with the heterocyclic structure, anti-CVB3 pharmacological researches and experiments of CVB3 replication inhibition effect of an analogue G19, the gramine analogue with the heterocyclic structure inhibits CPE (cytopathic effect) of CVB3 on a host cell Hep-2, increases cell survival rate, inhibits progeny virus production and viral nucleic acid generation, mainly inhibits an early replication stage of CVB3 viruses in the host cell and can be applied to preparation of the anti-CVB3 virus medicines.
Description
technical field [0001] The technical field of antiviral drugs of the present invention relates to the application of a Gramine analogue with a heterocyclic structure in the preparation of anti-CVB3 virus drugs. Background technique [0002] Coxsackievirus (Coxsaekievirus) is a member of the genus Enterovirus of the family Picornaviridae, and its infection can cause a variety of diseases, such as hand, foot and mouth disease, aseptic meningitis, encephalitis, myocarditis, epidemic Myositis pain, herpetic angina, etc. There are 29 serotypes of CV reported, which can be divided into two groups, A and B, according to their pathogenic characteristics and cell sensitivity to suckling mice, namely CVA (CVA1-22, 24) and CVB ( CVB1-6). CVBs infection is the most common, among which CVB3 is the most pathogenic type among the six serotypes of CVB, and is the main cause of viral myocarditis. According to the statistics of the US Centers for Disease Control and Prevention (CDC), CVB (...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K31/4439A61K31/444A61K31/428A61K31/4196A61K31/4155A61P31/14A61P35/00
CPCA61K31/4155A61K31/4196A61K31/428A61K31/4439A61K31/444
Inventor 魏艳红柯少勇杨自文石丽桥
Owner HUBEI UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com