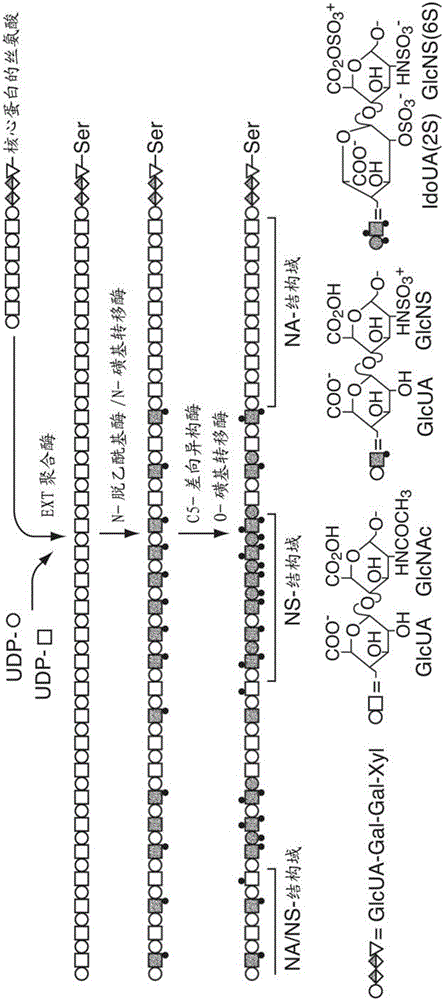
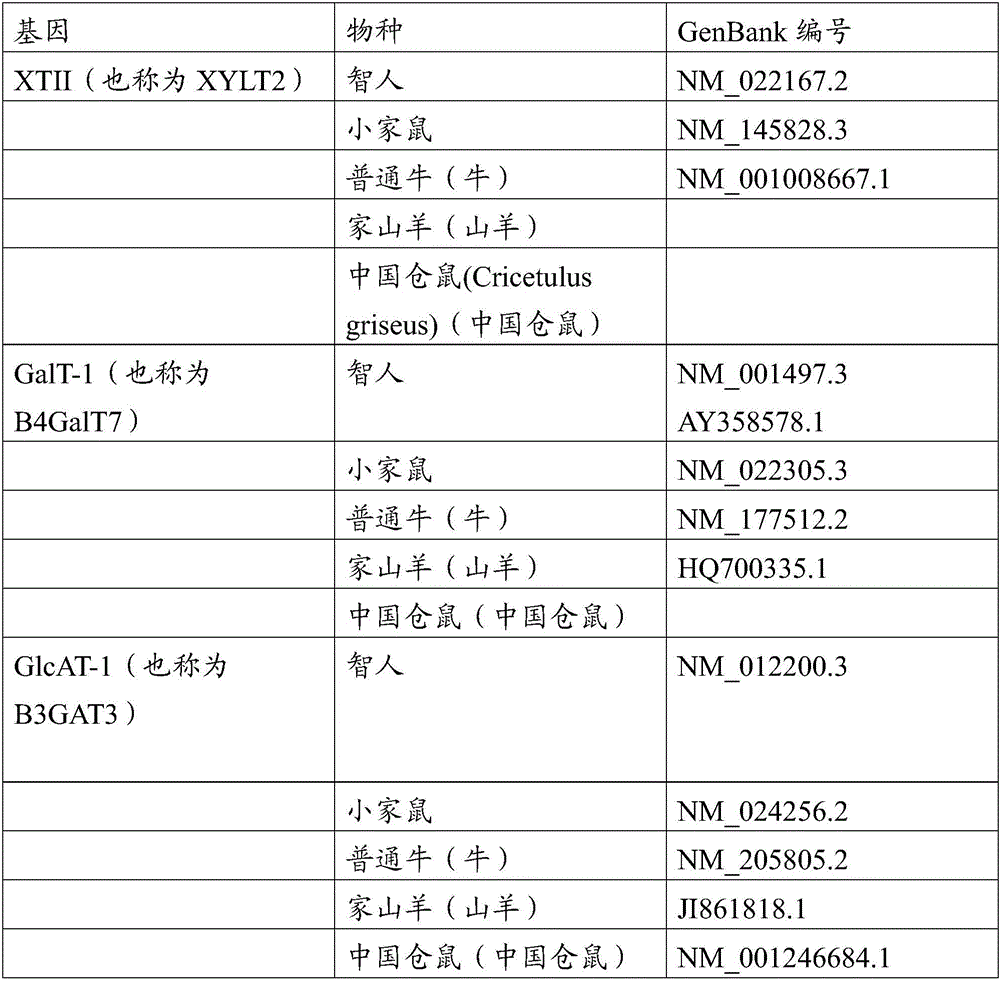
Transgenic production of heparin
A transgenic, heparin technology, applied in genetic engineering, microorganisms, biochemical equipment and methods, etc., can solve problems such as heparin pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0078] 1. Safety research
[0079] 1.1 Test the effect of heparin on mammary gland
[0080] The effect of heparin on the lactating mammary gland was evaluated. The level of goat antithrombin (AT) in the mammalian mammary gland is about 2 g / ml and it is found in 1% of the blood. Heparin can be infused into the mammary glands of lactating goats at levels corresponding to production levels of 1 mg / ml, 200 mg / ml and 50 mg / ml. Because the goat's mammary glands hold 1 liter of milk, heparin infusions (1 g, 200 mg, and 50 mg high molecular weight heparin infusions) can be given the day after milking and milk removal. Daily infusions of heparin can be given for one week or until toxicity is observed in the mammary gland or blood.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com