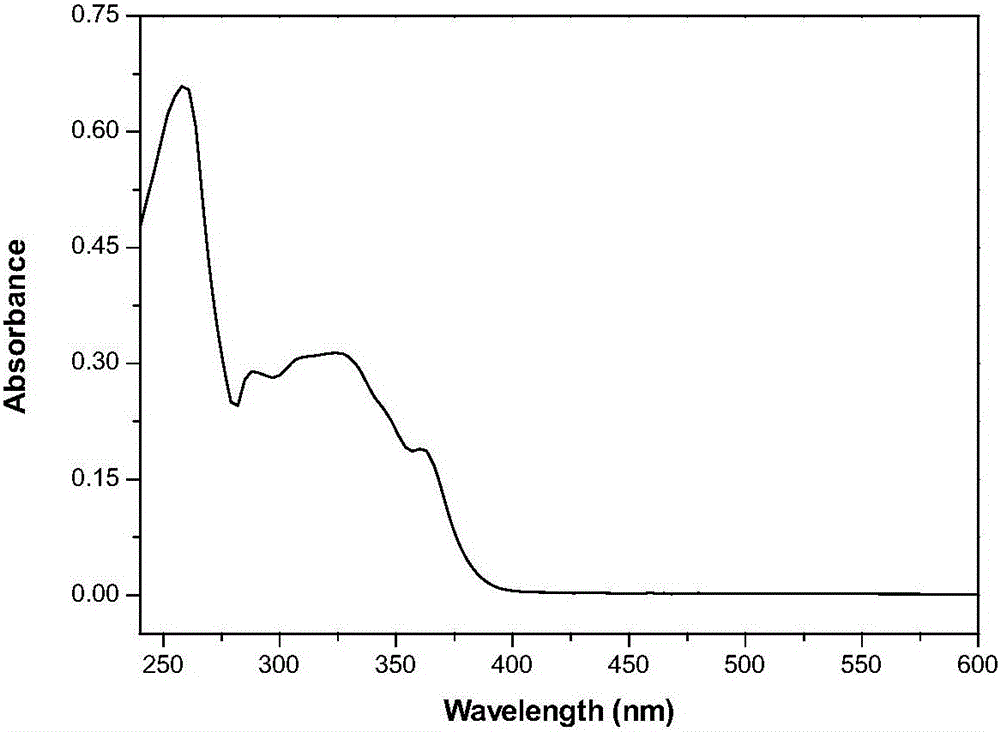
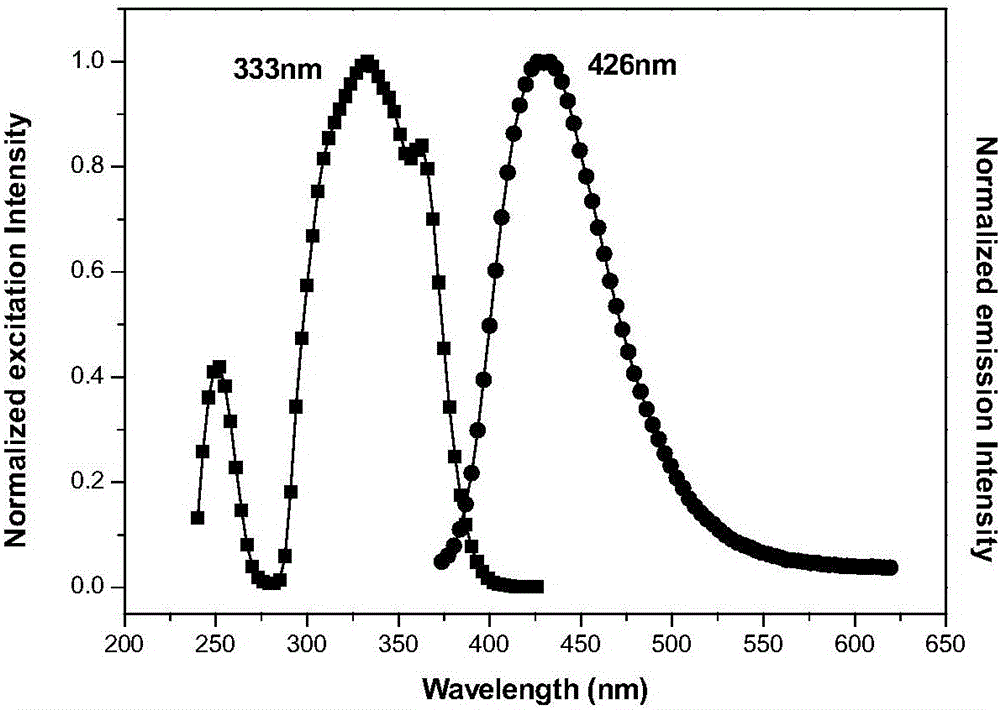
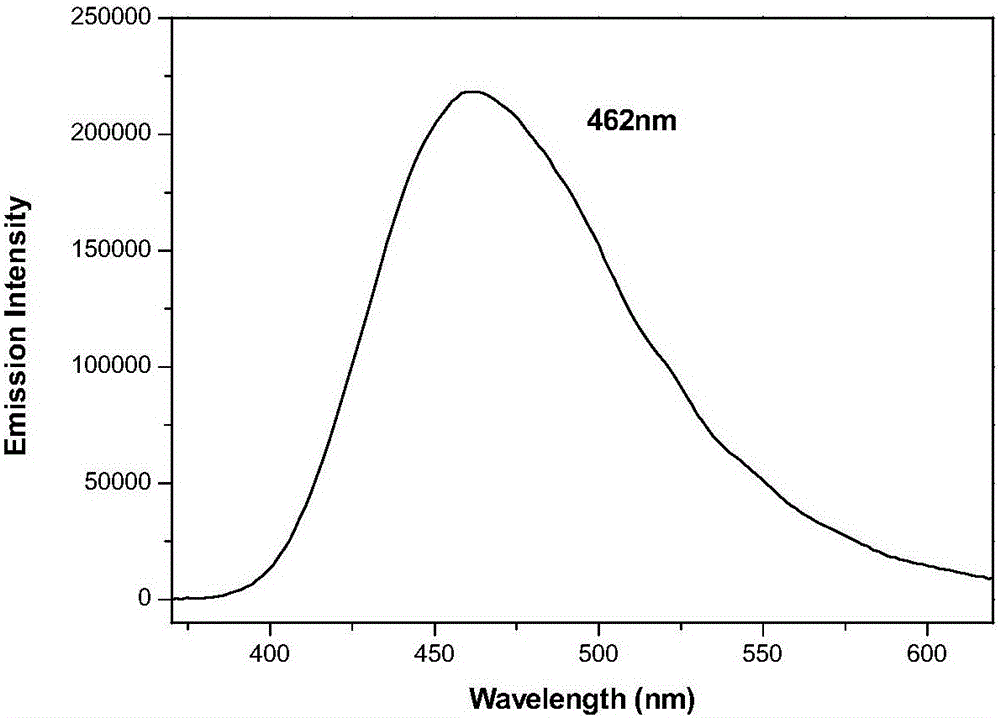
Decyl imidazole blue-light emission organic light-emitting material and preparation method thereof
A technology of decyl imidazole and luminescent materials, applied in luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the complex synthesis of imidazole compounds, poor material solubility, compatibility and processability, fluorescence quenching, etc. problems, achieving good optical properties, improving processability, and preventing concentration quenching
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: Synthesis of decyl imidazole blue light emitting organic luminescent material (compound I)
[0030] a. Synthesis of Intermediate (Compound II)
[0031] In a dry 250 ml flask, add 4-(1-n-decylphenanthrene[9,10-d]imidazol-2-yl)benzohydrazide (compound III) (0.01mol) and 2-diphenyl Phosphinebenzaldehyde (compound IV) (0.01mol) and 100 milliliters of absolute ethanol were stirred and refluxed for 5 hours, and the ethanol was evaporated under reduced pressure to precipitate a white solid. The crude product was recrystallized with ethanol to obtain an intermediate (compound II). The yield was 77%. The melting point is 200-202°C. ESI-MS m / z: 765.4 (M+H)+.
[0032] b. Synthesis of decyl imidazole blue light-emitting organic light-emitting materials (compound I)
[0033] The above-mentioned intermediate (compound II) was dissolved in 100 milliliters of dichloromethane, and under rapid stirring, 10 milliliters of 30% hydrogen peroxide was added dropwise at room te...
Embodiment 2
[0036] Synthesis of Decylimidazole Blue Light Emitting Organic Light-Emitting Material (Compound I)
[0037] a. Synthesis of Intermediate (Compound II)
[0038] In a dry 250 ml flask, add 4-(1-n-decylphenanthrene[9,10-d]imidazol-2-yl)benzohydrazide (compound III) (0.01mol) and 2-diphenyl Phosphinebenzaldehyde (compound IV) (0.011mol) and 100 milliliters of anhydrous methanol were stirred and refluxed for 7 hours, and methanol was evaporated under reduced pressure to precipitate a white solid. The crude product was recrystallized with ethanol to obtain an intermediate (compound II). The yield was 79%. ESI-MS m / z: 763.3 (M-H)-.
[0039] b. Synthesis of decyl imidazole blue light-emitting organic light-emitting materials (compound I)
[0040] The above intermediate (compound II) was dissolved in 100 ml of dichloromethane, and under rapid stirring, 20 ml of 30% hydrogen peroxide was added dropwise at room temperature, and the stirring reaction was continued for 5 hours at room ...
Embodiment 3
[0042] Synthesis of Decylimidazole Blue Light Emitting Organic Light-Emitting Material (Compound I)
[0043] a. Synthesis of Intermediate (Compound II)
[0044] In a dry 250 ml flask, add 4-(1-n-decylphenanthrene[9,10-d]imidazol-2-yl)benzohydrazide (compound III) (0.01mol) and 2-diphenyl Phosphinebenzaldehyde (compound IV) (0.011mol) and 100 milliliters of chloroform, stirred and refluxed for 6 hours, evaporated chloroform under reduced pressure, and precipitated a white solid, and the crude product was recrystallized with ethanol to obtain an intermediate (compound II). 75%. ESI-MS m / z: 765.4 (M+H)+.
[0045] b. Synthesis of decyl imidazole blue light-emitting organic light-emitting materials (compound I)
[0046] The above intermediate (compound II) was dissolved in 100 ml of dichloromethane, and under rapid stirring, 15 ml of 30% hydrogen peroxide was added dropwise at room temperature, and the stirring reaction was continued for 4 hours at room temperature, and then 50 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com