CDK2 inhibitor and applications thereof
A technology of inhibitors and uses, applied in the field of medicinal chemistry, can solve problems such as inability to phosphorylate substrates, inability to be activated, and loss of kinase activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
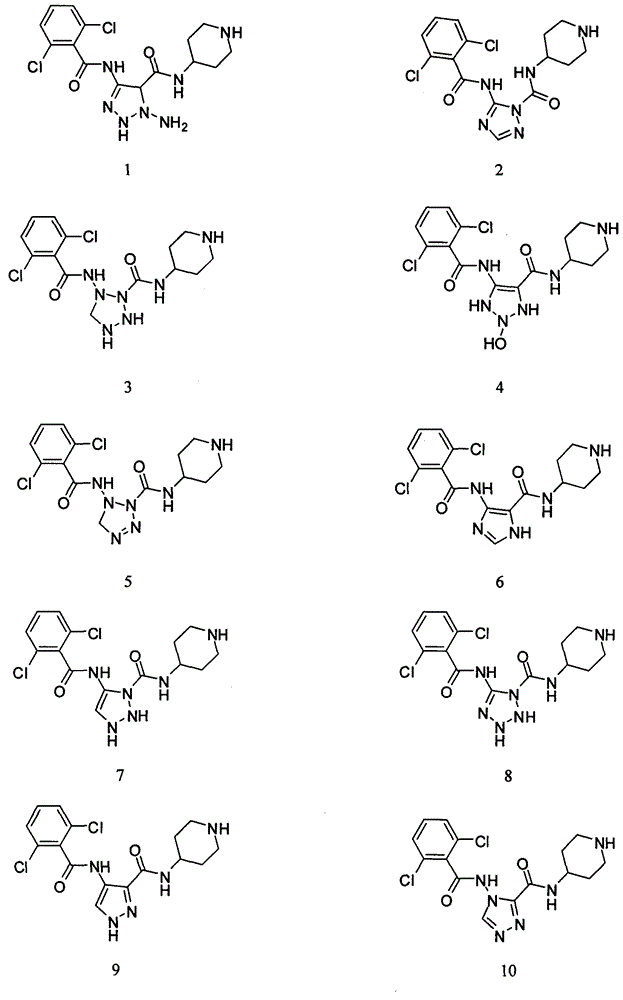
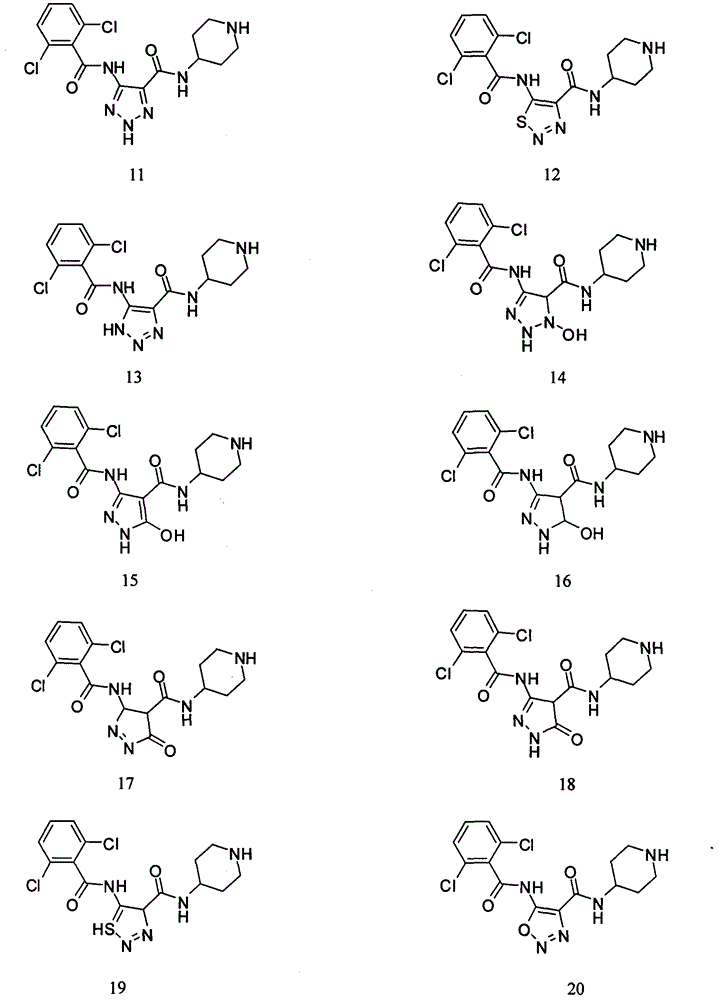
[0017] Experimental Study on the Inhibitory Activity of Compound 1~Compound 20 on CDK2
[0018] 1. CDK2 inhibitory activity test
[0019] 1) Experimental materials:
[0020] ·CDK2 / cyclin E Kinase
[0021] · Kinase-Glo Plus reagent, including Kinase-Glo Substrate and Kinase-Glo Buffer
[0022] Substrate: Histone H derived peptide: HDB, H09-19T
[0023] Assay Buffer: 25mM HEPES, 10μM MgCl2, 0.01% Triton X-100, 100μm / ml BSA, 2.5mM DTT, pH7.4
[0024] ·ATP
[0025] · White opaque 384-well plate
[0026] 2) Experimental steps:
[0027] · All the compounds to be tested were dissolved in DMSO to prepare a stock solution with a concentration of 100 μM.
[0028] ·Take the compound to be tested and dilute it to 20 μL with assaybuffer to obtain a compound concentration of 10 μM.
[0029] 4*ATP: ATP is diluted with assay buffer to obtain 4*working solution.
[0030] 4*Substrate: The substrate is diluted with assay buffer to obtain 4*working solution.
[0031] ·2.5*CDK2 / cyclin E ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com