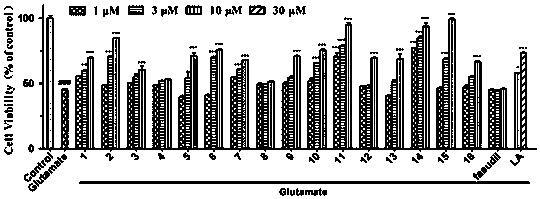
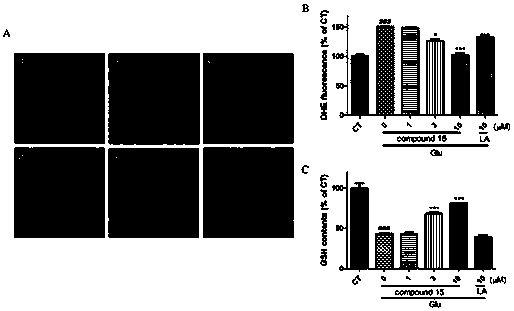
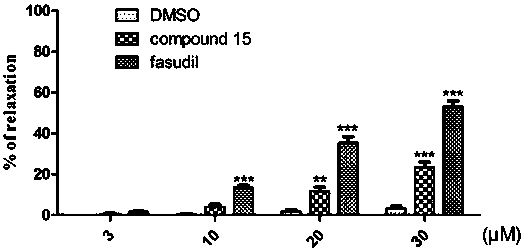
Compounds for treating central nervous system degenerative diseases and their applications
A degenerative disease and central nervous system technology, applied in the field of medicine, can solve problems such as single target and lipoic acid instability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 - 5-(1,2-dithiocyclopent-3-yl)-N-(4-(pyridin-4-yl)phenyl)pentanamide (1)
[0043]
[0044] Compound 1 was synthesized according to the above-mentioned synthetic route 1 to obtain a light yellow solid with a total yield of 52% in three steps. 1 H NMR (400 MHz, CDCl 3 ) δ 8.62 (d, J = 4.7 Hz, 2H), 7.96 (s, 1H), 7.68 (d, J = 8.1Hz, 2H), 7.60 (d, J = 8.4 Hz, 2H), 7.48 (d, J = 5.2 Hz, 2H), 3.64 – 3.49 (m,1H), 3.22 – 3.04 (m, 2H), 2.53 – 2.33 (m, 3H), 1.90 (dt, J = 19.7, 6.8 Hz,1H), 1.84 – 1.62 (m, 4H), 1.61 – 1.43 (m, 2H). 13 C NMR (100 MHz, CDCl 3 ) δ171.4, 150.1, 147.7, 139.2, 133.4, 127.6, 121.3, 120.2, 56.4, 40.3, 38.5,37.4, 34.7, 28.9, 25.2. ESI-HRMS for [C 19 h 23 N 2 OS 2 ] + , calcd: 359.1246; found: 359.1232. Purity: 100% (HPLC). .
Embodiment 2
[0045] Example 2 - [5-(1,2-dithiocyclopentane-3-yl)-N-(2-fluoro-4-(pyridin-4-yl)phenyl)pentanamide (2)
[0046]
[0047] Compound 2 was synthesized according to the above-mentioned synthetic route 1 to obtain a light yellow solid, and the total yield of 3 steps was 32.8%. 1 H NMR (400 MHz, CDCl 3 ) δ 8.65 (d, J = 6.0 Hz, 2H), 8.48 (t, J = 8.2 Hz, 1H), 7.51 –7.35 (m, 5H), 3.66 – 3.53 (m, 1H), 3.25 – 3.06 (m, 2H), 2.57 – 2.37 (m, 3H),1.93 (td, J = 13.6, 6.9 Hz, 1H), 1.87 – 1.72 (m, 4H), 1.62 – 1.47 (m, 2H). 13 CNMR (100 MHz, CDCl 3 ) δ 170.16 (s), 151.50 (d, J = 243.8 Hz), 149.33 (s), 145.51 (d, J = 1.8 Hz), 133.04 (d, J = 7.5 Hz), 126.22 (d, J = 10.5 Hz), 122.13 (d, J = 3.0 Hz), 121.10 (s), 120.13 (s), 112.21 (d, J = 20.7 Hz), 55.32 (s), 39.24 (s), 37.49 (s), 36.43 (s), 33.64 (s), 27.80 (s), 24.07 (s). ESI-HRMS for [C 19 h 22 FN 2 OS 2 ] + ,calcd: 377.1158; found: 377.1151. Purity: 99% (HPLC).
Embodiment 3
[0048] Example 3 - 5-(1,2-dithiocyclopent-3-yl)-N-(2-methoxy-4-(pyridin-4-yl)phenyl)pentanamide (3)
[0049]
[0050] Compound 3 was synthesized according to the above-mentioned synthetic route 1 to obtain a light yellow solid, and the total yield of the three steps was 22%. 1 H NMR (400 MHz, CDCl 3 ) δ 8.62 (dd, J = 4.5, 1.6 Hz, 2H), 8.50 (d, J = 8.4 Hz, 1H),7.83 (s, 1H), 7.47 (dd, J = 4.5, 1.6 Hz, 2H), 7.29 – 7.23 (m, 1H), 7.11 (d, J= 1.8 Hz, 1H), 3.97 (s, 3H), 3.64 – 3.52 (m, 1H), 3.24 – 3.06 (m, 2H), 2.54 –2.39 (m , 3H), 1.97 – 1.85 (m, 2H), 1.83 – 1.69 (m, 4H), 1.60 – 1.47 (m, 2H). 13 C NMR (100MHz, CDCl 3 ) δ 170.0, 149.2, 147.1, 147.0, 132.3, 127.7, 120.3, 119.0, 107.3, 75.7, 55.4, 54.9, 39.3, 37.5, 36.7, 33.7, 27.8, 24.2. + ,calcd: 389.1352; found: 389.1350. Purity: 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com