Eps8-SH3 structural domain-based dual antitumor polypeptide
An eps8-sh3, anti-tumor technology, applied in the field of preparation of anti-tumor drugs, can solve the problems of unsatisfactory inhibition of tumor cell proliferation and low inhibition rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
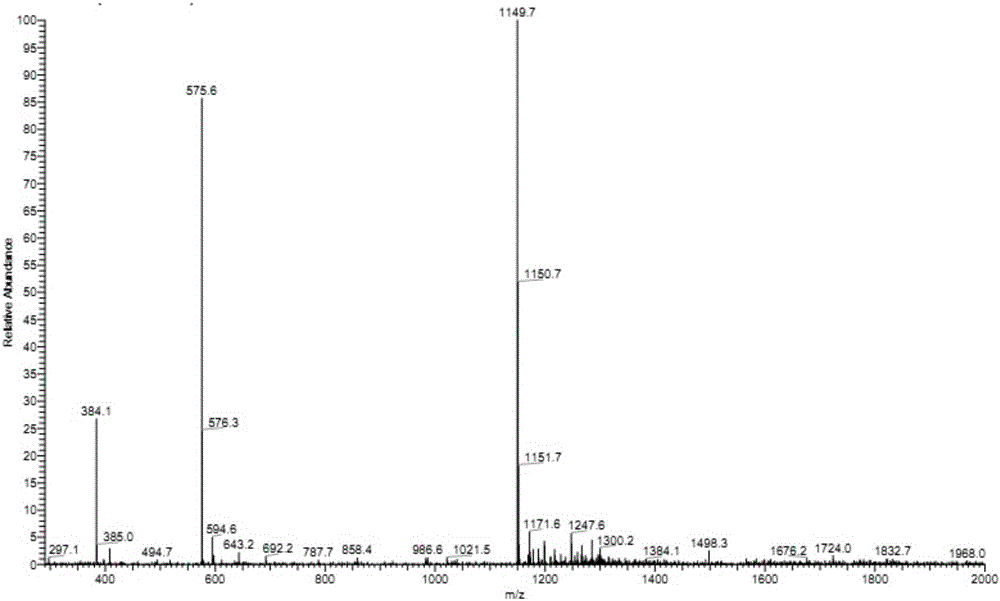
[0023] Example 1 (screening of Eps8-SH3 binding domain polypeptides)
[0024] The anti-tumor polypeptide derived from the Eps8-SH3 domain of the present invention is based on the primary structure of the antigen, using immunoinformatics means, and using the online biological software SYFPEITHI (http: / / www.syfpeithi.de / bin / MHCServer .dll / EpitopePrediction.htm) (the algorithm is described in Rammensee H, et al. Immunogenetics 1999; 50(3-4):213-219.) and BIMAS (http: / / www-bimas.cit.nih.gov / molbio / hla_bind / ) (this algorithm is described in Parker KC, et al. J Immunol 1994; 152 (1): 163-175.) for the HLA-A of the SH3 domain of the Eps8 molecule * The 2402 restriction was used for predictive analysis, and the polypeptide whose amino acid sequence was shown as SEQ ID No: 1 was screened out, and recorded as Eps8-534. The polypeptide shown in SEQ ID No: 1 was synthesized by Hangzhou Zhongpei Biochemical Co., Ltd. according to the sequence provided by the inventor, using the Fmoc soli...
Embodiment 2
[0026] Example 2 [Induction of sensitized antigen-presenting cells (APC) in vitro]
[0027] Using peripheral blood mononuclear cell-derived dendritic cells (DCs) as APCs, DCs were induced in vitro referring to elsewhere (NakaharaS, et al. Cancer Res 2003 Jul 155, 63(14):4112-8). Specifically, healthy volunteers (HLA-A * 2402 positive) isolated mononuclear cells (PBMCs) from peripheral blood, and isolated original DC by the adherent method. 1000U / ml interleukin 4 (IL-4) culture medium. On day 7 of culture, cytokine-induced DCs were pulsed with each of the synthetic peptides (20 µg / ml) for 3 hours in a medium containing 3 µg / ml β2-microglobulin. The resulting cells expressed DC-associated molecules such as CD80, CD83, CD86 and HLA-II molecules on the cell surface (data not shown).
Embodiment 3
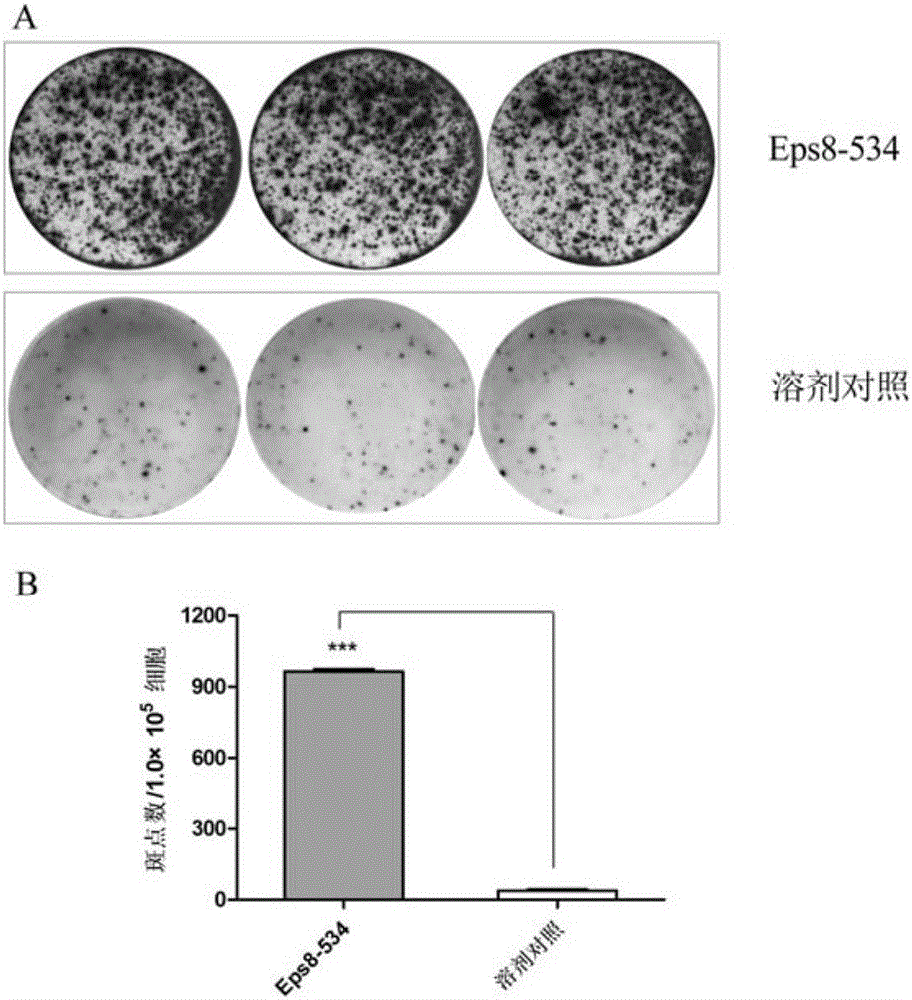
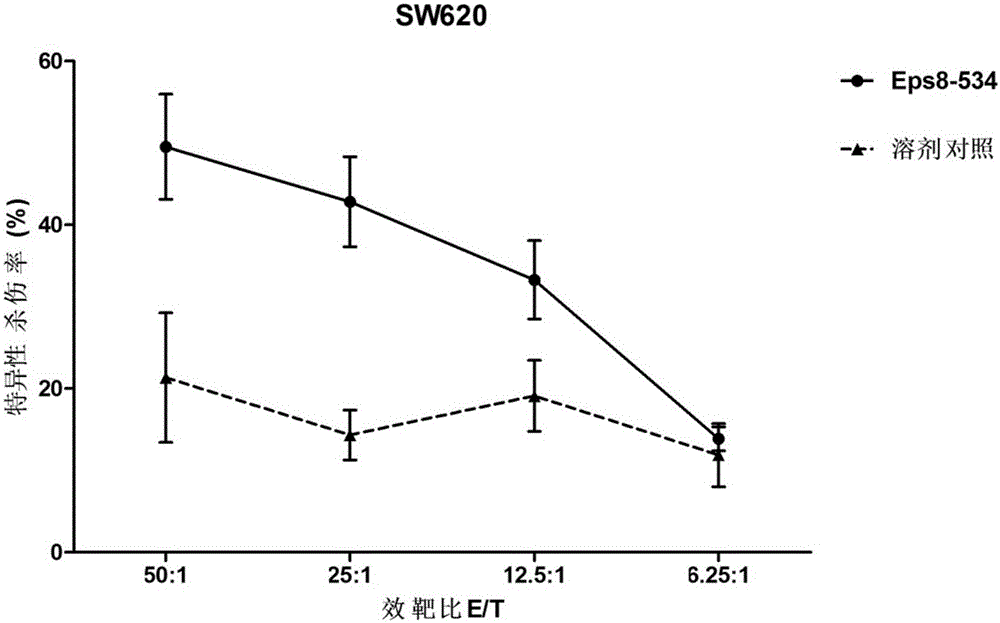
[0028] Example 3 (induction of CTL in vitro)
[0029] The DCs pulsed with the polypeptide were inactivated with mitomycin C (MMC) (30 μg / ml, 30 min), and mixed with autologous CD8 at a ratio of 1:20. + T cells (obtained by positive selection from CD8 positive isolation kit) were mixed and cultured in medium containing IL-7 10ng / ml. On day 3, IL-2 was added to the medium to a final concentration of 20 U / ml. On days 7 and 14, T cells were further stimulated with peptide-pulsed, inactivated autologous DCs. On day 21, cells were collected to detect CTL function (OlsonBM, et al. Cancer Immunol Immunother 2011; 60(6): 781-792; Andersen RS, et al. Cancer Immunol Immunother 2011; 60(2): 227-234.).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com