A kind of preparation method of polylactic acid polyol with low acid value
A technology of polylactic acid polyol and low acid value, which is applied in the field of preparation of low acid value polylactic acid polyol, can solve the problems affecting the reactivity of polylactic acid polyol and high acid value of polylactic acid polyol, and achieve low production cost, The effect of enhancing reactivity and no discharge of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0017] A preparation method of low acid value polylactic acid polyols, comprising the following steps (the following parts are parts by weight):
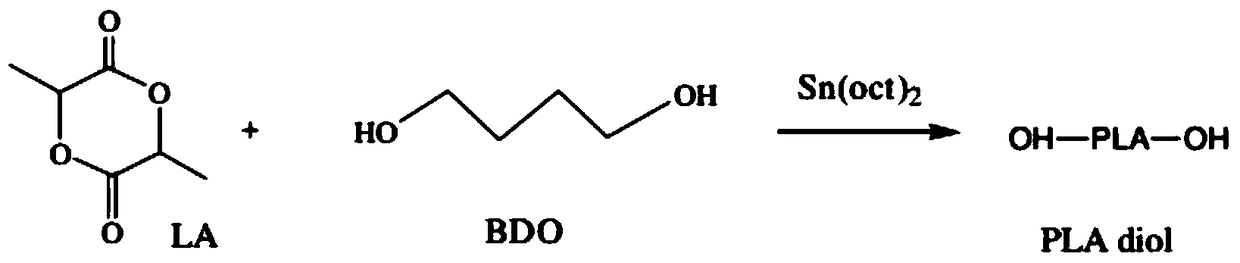
[0018] Preparation of polylactic acid: Add 6.63 to 70.32 parts by weight of 1,4-butanediol, 216.02 to 432.67 parts by weight of lactide, and 0.11 to 0.60 parts by weight of catalyst in a round bottom beaker, vacuumize and ventilate with nitrogen , stirred and heated to 135-175°C under nitrogen protection, and reacted at 135-175°C for 4-28 hours to obtain polylactic acid with a theoretical molecular weight of 500-4000 and an acid value of 3.50-14.10. Preferably, the catalyst is ferric chloride, aluminum chloride, butyllithium, sodium alkoxide, potassium alkoxide stannous octoate, tin tetrachloride, stannous chloride, stannous acetate, tin oxide or any combination thereof. The chemical reaction equation of above-mentioned reaction is as follows:
[0019]
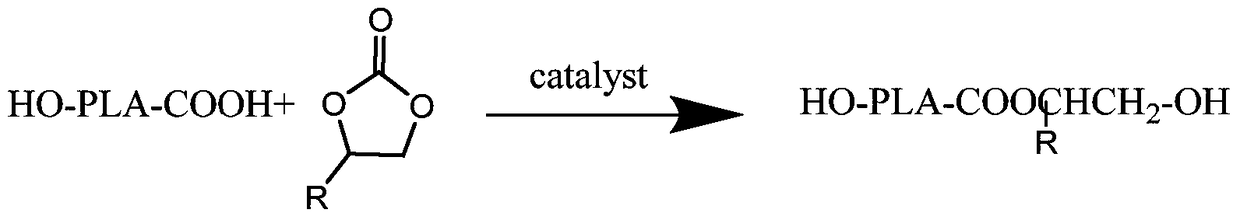
[0020] Preparation of polylactic acid polyols with low acid value: add 50.5...
Embodiment 1
[0028] A preparation method of low acid value polylactic acid polyols, comprising the following steps (the following parts are parts by weight):
[0029] The preparation of polylactic acid with a theoretical molecular weight of 500: add 47.20g of 1,4-butanediol, 216.02g of lactide, and 0.11g of stannous octoate in a round-bottomed beaker, vacuumize and replace with nitrogen for 3 times. Stir and heat up to 140°C under protection, and react at 140°C for 24 hours to obtain 261.56 g of polylactic acid with a theoretical molecular weight of 500 and an acid value of 3.50.
[0030] Modification of polylactic acid: Add 70.32g of polylactic acid prepared above, 0.15g of tetraethylammonium bromide, and then add 1.54g of ethylene carbonate in a round bottom flask, vacuumize and replace with nitrogen for 3 times, and protect Stir under the conditions, raise the temperature to 140° C., and react at 140° C. for 1.0 hour to obtain a polylactic acid polyol with an acid value of 2.20.
Embodiment 2
[0032] A preparation method of low acid value polylactic acid polyols, comprising the following steps (the following parts are parts by weight):
[0033] The preparation of polylactic acid with a theoretical molecular weight of 1000: add 28.40g 1,4-butanediol, 288.65g lactide, and 0.14g stannous octoate to a round-bottomed beaker, vacuumize and replace with nitrogen for 3 times. Stir and heat up to 140°C under protection, and react at 140°C for 24 hours to obtain 301.33g of polylactic acid with a theoretical molecular weight of 1000 and an acid value of 4.00.
[0034] Modification of polylactic acid: add 52.20g of polylactic acid prepared above, 0.43g of tetraethylammonium bromide, and then add 1.46g of ethylene carbonate in a round bottom flask, vacuumize and replace with nitrogen for 3 times, under nitrogen protection conditions Stir under low temperature, increase the temperature to 165°C, and react at 165°C for 6.0 hours to obtain polylactic acid polyol with low acid value...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com