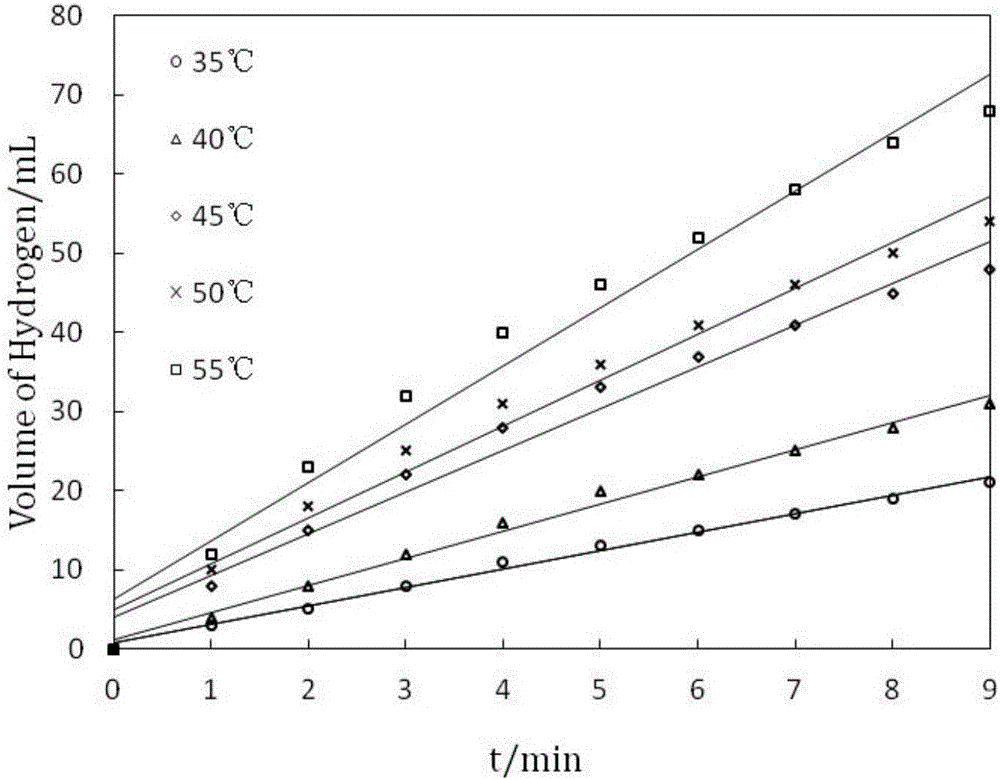
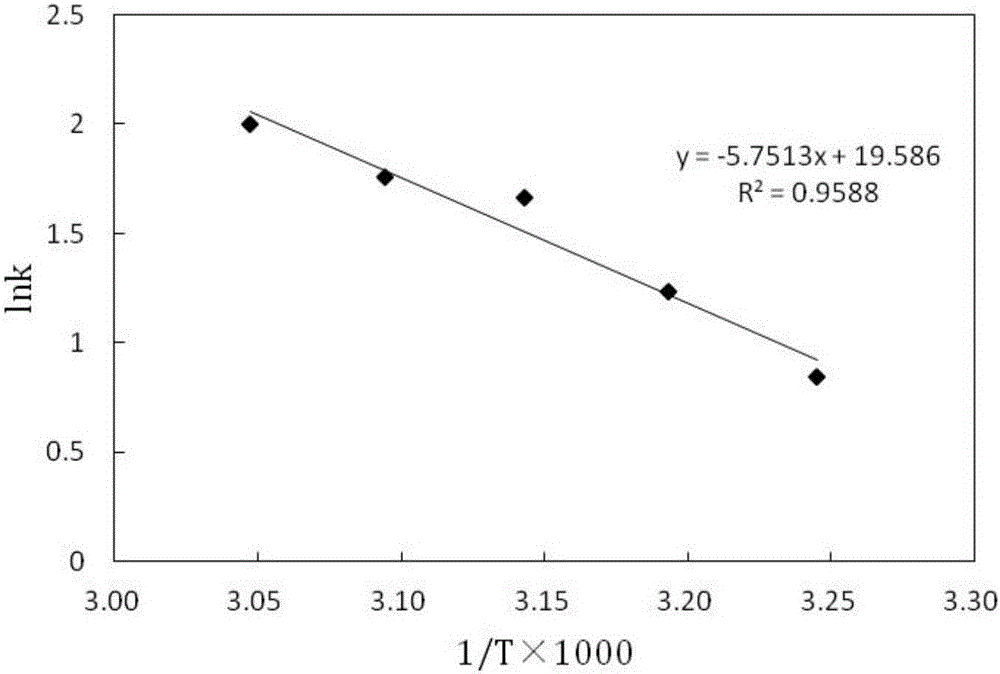
Application of terpolymer nanosphere loaded Ni-B catalyst to sodium borohydride catalysis and hydrolysis hydrogen generation reaction
A technology for catalyzing sodium borohydride and terpolymers, which is applied in the field of materials science, can solve the problems of catalyst agglomeration, low catalytic activity, and poor reusability, and achieve high hydrogen yield, high reactivity, and easy separation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation of Ni-B nanocatalyst
[0036] (1) Preparation of terpolymer nanospheres:
[0037] Weigh 1.13g N-isopropylacrylamide, 0.104g 2-acrylamide-2-methylpropanesulfonic acid, 0.254g N-tert-butylacrylamide, 0.046g N,N'-methylenebisacrylamide In a 100mL three-neck flask, add 45mL of distilled water, stir and dissolve at 70°C under a nitrogen atmosphere, then add 5mL of an aqueous solution containing 0.053g of potassium persulfate, continue to pass nitrogen for 10min, then close it, and seal the reaction for 5h.
[0038] (2) Pretreatment of terpolymer nanospheres:
[0039] Take 15mL of the terpolymer emulsion prepared in the above steps and disperse it in 35mL of a solution containing 1.39g of nickel sulfate, stir for 15h, centrifuge, and repeatedly centrifuge with water, then disperse the solid in 40mL of water, add 10mL of an aqueous solution containing 0.1g of sodium borohydride, Stir the reaction as a stock solution.
[0040] (3) Preparation of Ni-B catalyst:
...
Embodiment 2
[0043] The preparation method is the same as in Example 1, except that in the preparation of the Ni-B catalyst, the amount of sodium borohydride used in the nickel plating solution is 0.1 g.
Embodiment 3
[0045] The preparation method is the same as in Example 1, except that in the preparation of the Ni-B catalyst, the amount of sodium borohydride used in the nickel plating solution is 0.2 g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com