Application and method of α-chymotrypsin in synthesizing bisindolyl-indolin-2-one compounds
A technology of chymotrypsin and ketone compounds, applied in the field of chemistry, to achieve the effects of easy operation, rich research and high yield in the catalytic process
Active Publication Date: 2019-10-22
SOUTHWEST UNIV
View PDF0 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Interestingly, certain bisindolyl-indolin-2-one derivatives have been reported to act strongly on a range of tumor cells but not on normal cells
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment Construction
[0029] The following will describe in detail in combination with preferred embodiments. For the experimental methods that do not specify specific conditions in the examples, usually follow the conventional conditions or the conditions suggested by the manufacturer.
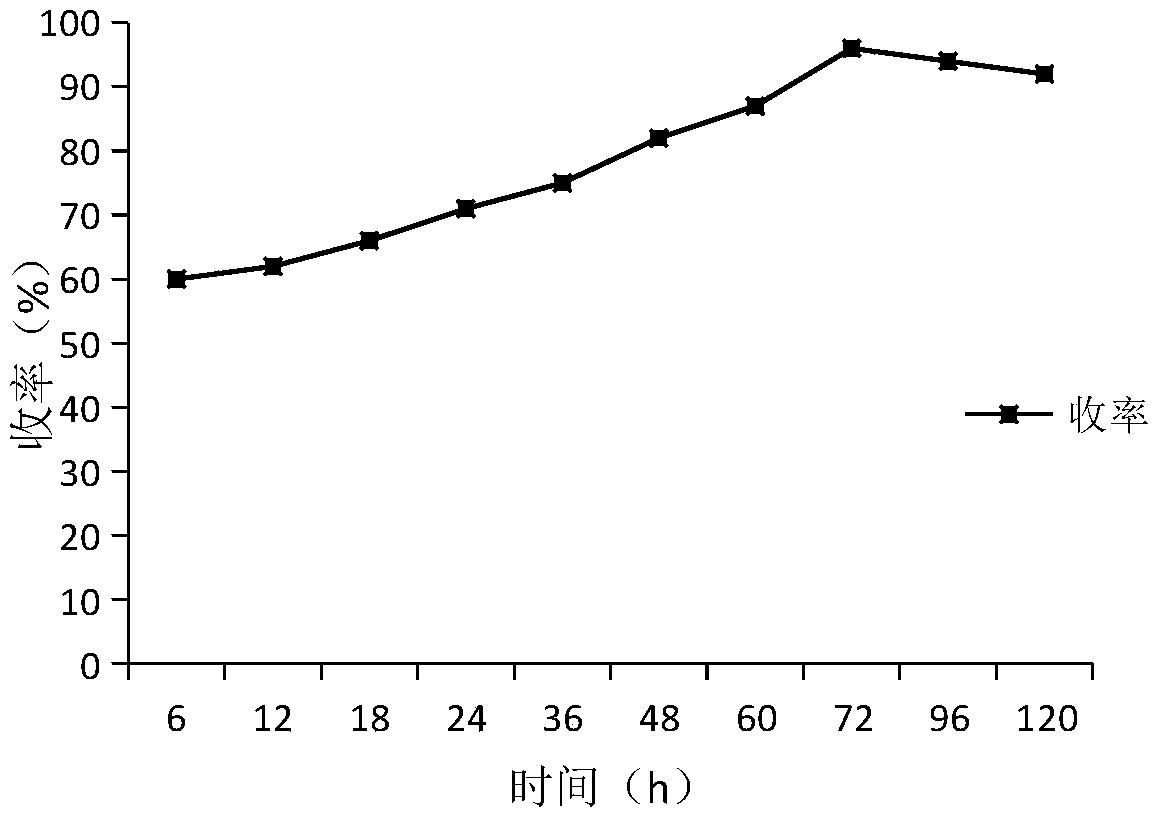
[0030] 1. Screening of solvents for the synthesis of bisindolyl-indolin-2-one by Friedel-Crafts reaction catalyzed by α-chymotrypsin
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Login to View More
Abstract
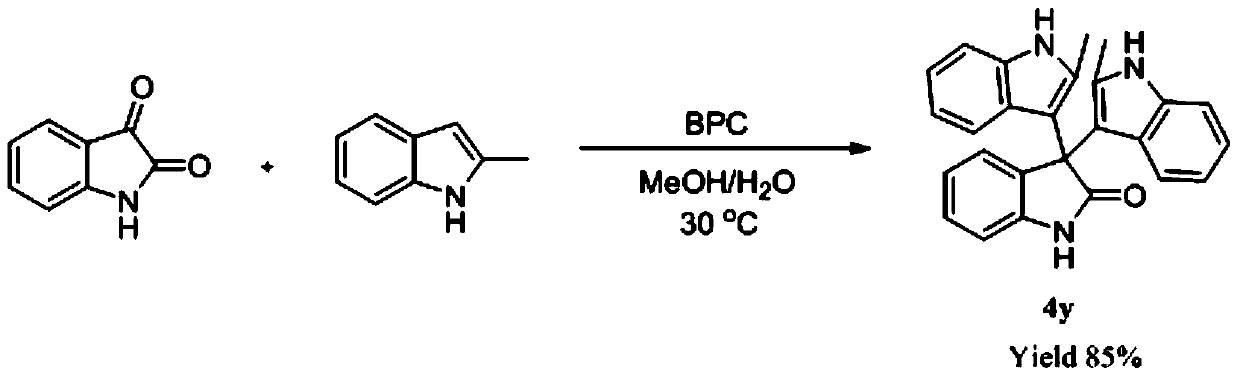
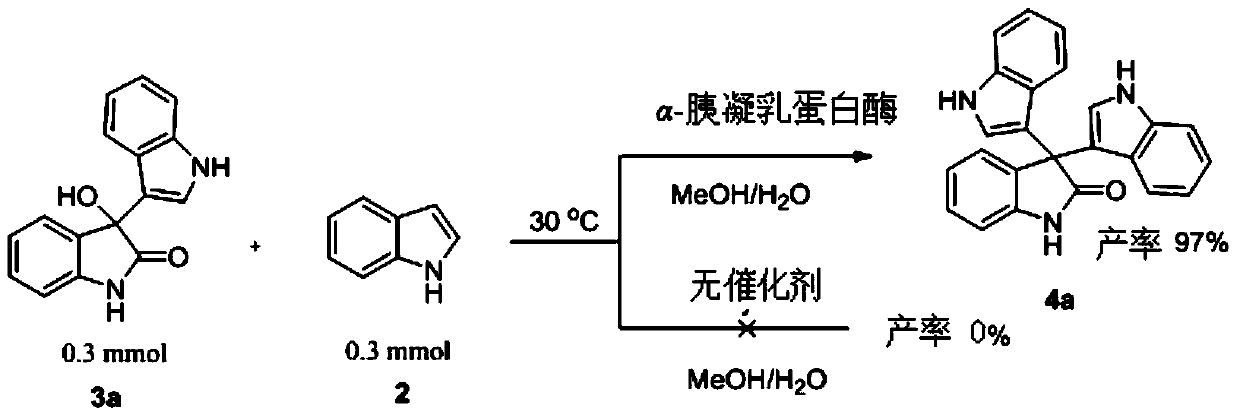
The invention discloses application of alpha-chymotrypsin catalyzed friedel-crafts reaction in synthesizing a bis(indolyl)-indoline-2-ketone compound and a method thereof. A general formula 1 and a general formula 2 can produce the bis(indolyl)-indoline-2-ketone compound in methanol or ethanol solution under catalysis of the alpha-chymotrypsin. The catalytic process of the method is simple and easy in operation, the highest yield can reach 97%, excellent substrate adaptability is exhibited, research on multifunctionality of enzyme is enriched, and also an efficient synthesis approach for synthesizing the bis(indolyl)-indoline-2-ketone compound is provided.
Description
technical field [0001] The invention belongs to the field of chemistry, and relates to the application of the Friedel-Crafts reaction catalyzed by α-chymotrypsin in the synthesis of bisindolyl-indolin-2-ketone compounds, and also relates to the preparation of bisindolyl-indoline Method for indol-2-ones. Background technique [0002] Since indole was discovered in 1866 because of its special structural characteristics, it has become a research hotspot in many fields including medicine, spices, agricultural chemicals, pigments and material science. These works clearly show the role of indole in organic synthesis. occupy an important position. [0003] Bisindole structure exists in many drugs. Such derivatives have been proven to have a wide range of biological activities, such as anti-plasmodium, anti-virus, anti-bacterial, anti-tumor, anti-convulsant, treatment of Parkinson's disease and effective inhibitor of SARS coronavirus 3CL protease. Among them, bisindolyl-indolin-2...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C12P17/16
Inventor 官智薛靖文
Owner SOUTHWEST UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com