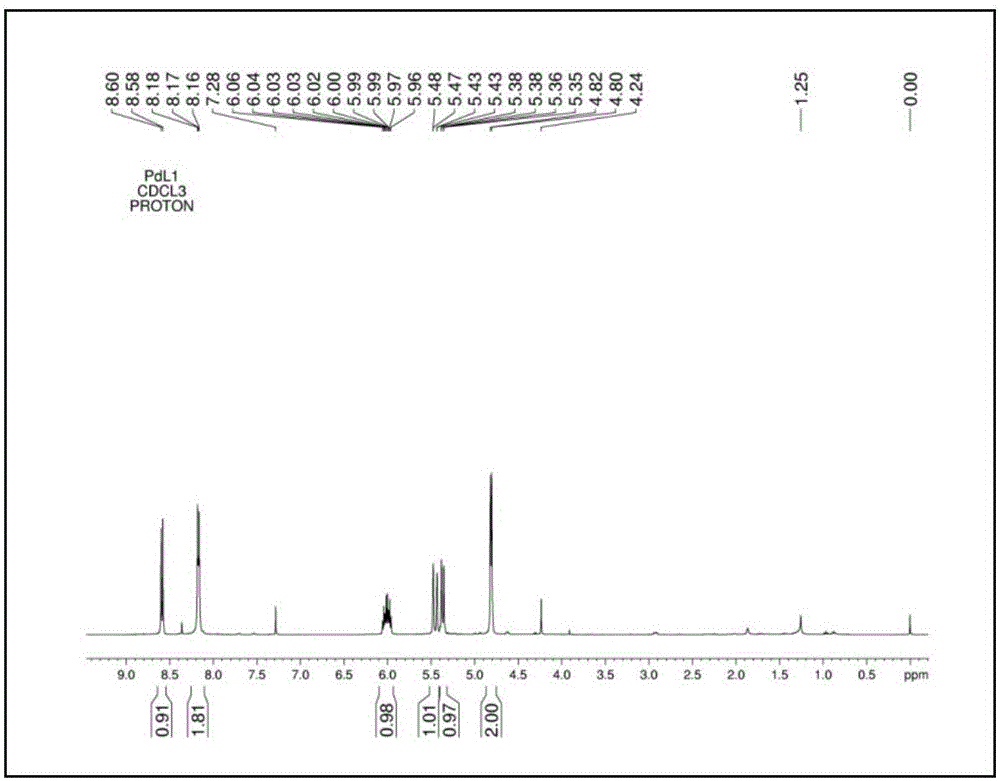
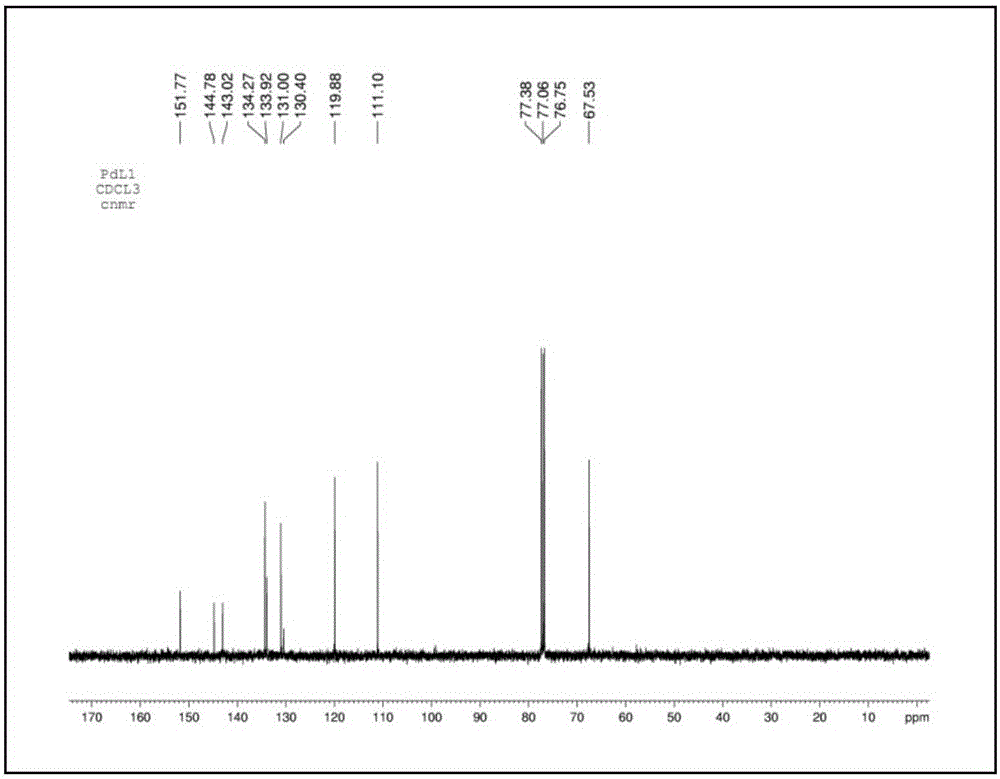
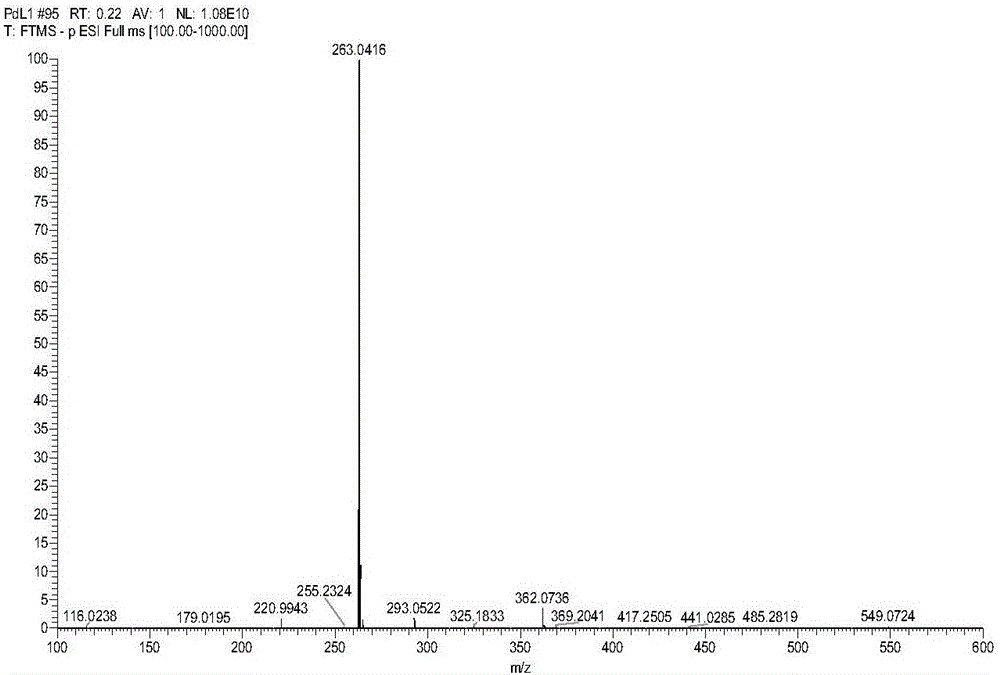
Pd (0) fluorescence probe based on 7-nitro-1,2,3-benzoxadiazole and preparation method and application thereof
A technology of nitrofenfurazan and fluorescent probes, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., to achieve the effects of low detection limit, simple synthesis process and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (1) Intermediate 4-amino-7-nitro-1,2,3-benzofurazan (NBD-NH 2 ) preparation
[0042] Wrap a 100 mL single-necked round-bottom flask with tin foil, add ammonia (1 mL, 20 mmol) solution dropwise to a methanol solution containing 1 mmol 4-chloro-7-nitrobenzofurazan (NBD-Cl), 4 The concentration of the methanol solution of -chloro-7-nitrobenzofurazan (NBD-Cl) is 40%. After stirring at room temperature for 20 hours, after fully reacting, the solid is obtained by distillation under reduced pressure. The solid is dissolved in 8 mL of ethyl acetate, and the Ethyl acetate and petroleum ether were used as eluents (volume ratio 1:1), and 108 mg of a tan solid was obtained by silica gel column chromatography, which was the intermediate NBD-NH 2 The product, yield 60%.
[0043] (2) Preparation of Pd(0) fluorescent probe
[0044] Under dark conditions, the intermediate NBD-NH 2 (180 mg, 1 mmol) and triethylamine (270 μL, 2 mmol) were mixed in 10 mL of dichloromethane solution, a...
Embodiment 2
[0047] The preparation method of Pd (0) fluorescent probe, the steps are as follows:
[0048] (1) Intermediate 4-amino-7-nitro-1,2,3-benzofurazan (NBD-NH 2 ) preparation
[0049] Wrap a 100 mL single-necked round-bottom flask with tinfoil, add 15 mmol ammonia solution dropwise to a methanol solution containing 1 mmol 4-chloro-7-nitrobenzofurazan (NBD-Cl), NBD-Cl in methanol The concentration of the solution is 60%. After stirring at room temperature for 25 hours, after sufficient reaction, the solvent is removed under reduced pressure, and the solid is dissolved in 6 mL of ethyl acetate, and ethyl acetate and petroleum ether are used as eluents (volume ratio: 1:5) , separated by silica gel column chromatography to obtain 100 mg of tan solid, which is the intermediate NBD-NH 2 The product, yield 50%.
[0050]
[0051] (2) Preparation of Pd(0) fluorescent probe
[0052] Under dark conditions, 1 mmol intermediate NBD-NH 2 Mix with 1.5mmol triethylamine in 10 mL dichlorome...
Embodiment 3
[0056] The preparation method of Pd (0) fluorescent probe, the steps are as follows:
[0057] (1) Intermediate 4-amino-7-nitro-1,2,3-benzofurazan (NBD-NH 2 ) preparation
[0058] Wrap a 100 mL single-necked round-bottom flask with tinfoil, add 30 mmol ammonia solution dropwise to a methanol solution containing 1 mmol 4-chloro-7-nitrobenzofurazan (NBD-Cl), 4-chloro-7 -The concentration of the methanol solution of nitrobenzenefurazan (NBD-Cl) is 70%. After stirring at room temperature for 30 hours, after sufficient reaction, the solvent is removed under reduced pressure, and the solid is dissolved in 10 mL of ethyl acetate. Ether was used as the eluent (volume ratio 1:10), and 120 mg of a tan solid was obtained by silica gel column chromatography, which was the intermediate NBD-NH 2 The product, yield 80%.
[0059] (2) Preparation of probes
[0060] Under dark conditions, 1 mmol intermediate NBD-NH 2 Mix with 5 mmol triethylamine in 10 mL dichloromethane solution, dropwise ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap