Schiff base compound based on tetraphenylethylene and mandelonitrile as well as preparation method and application of Schiff base compound
A technology of tetraphenylethylene and tetraphenylethylene, which is applied in the field of analysis and detection, and can solve the problems of low fluorescence efficiency and inability to realize the application of red fluorescent probes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Embodiment 1: two tetraphenylethylene maleonitrile synthesis (TPE-DMNS)
[0079]
[0080] Reflux 1g of tetrastyryl salicylaldehyde and appropriate amount of diaminomaleonitrile hydrazine hydrate under 25ml for 4h (adding acetic acid as catalyst), after cooling, remove ethanol under reduced pressure and then extract. After the organic phase is dried, it is separated by a column to obtain light yellow 0.8g of green solid, namely TPE-DMNS structure. MALDI-TOF (m / z): [M+]calcd.C 58 h 40 N 4 o 2 , 824.3151; found, 824.4532. Anal Calc. for C 20 h 14 N 2 O: C, 84.44; H, 4.89; N, 6.79; O, 3.88. Found: C, 84.64; H, 4.84; N, 6.74; O, 3.86.
Embodiment 2
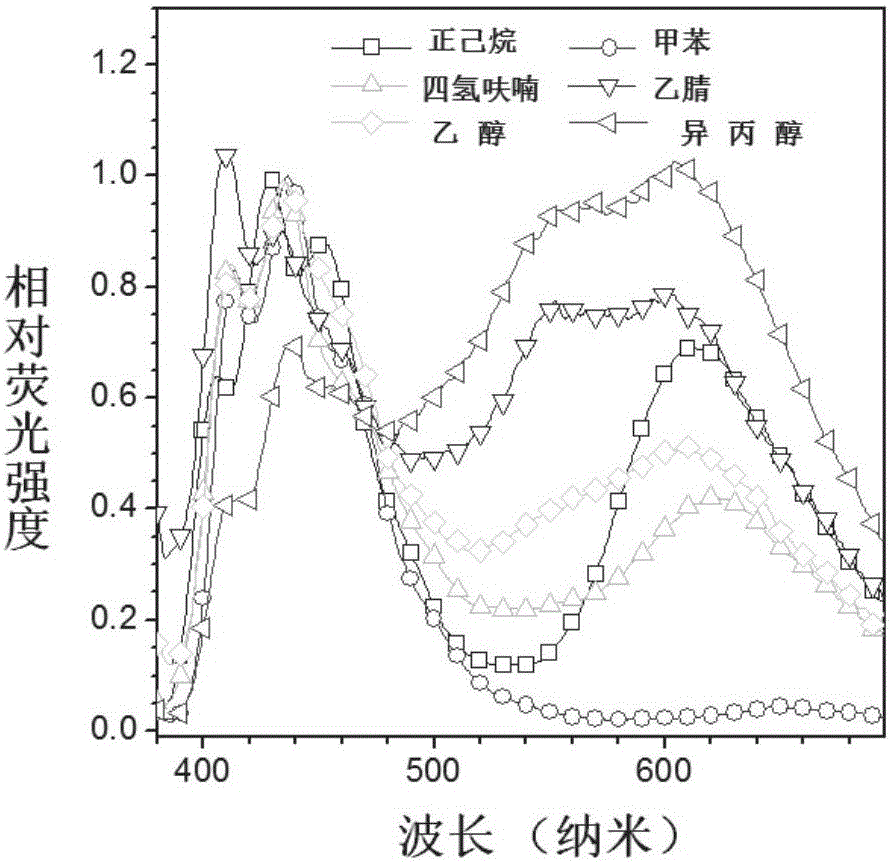
[0081] Example 2: ESIPT (intramolecular proton transfer) properties of TPE-DMNS
[0082] Such as figure 1 Shown is the fluorescence spectrum of TPE-DMNS under different polarities. With the change of polarity, the ratio of alcohol emission (near 432nm) and ketone emission (near 615nm) of TPE-DMNS changes significantly, which is typical ESIPT launch.
Embodiment 3
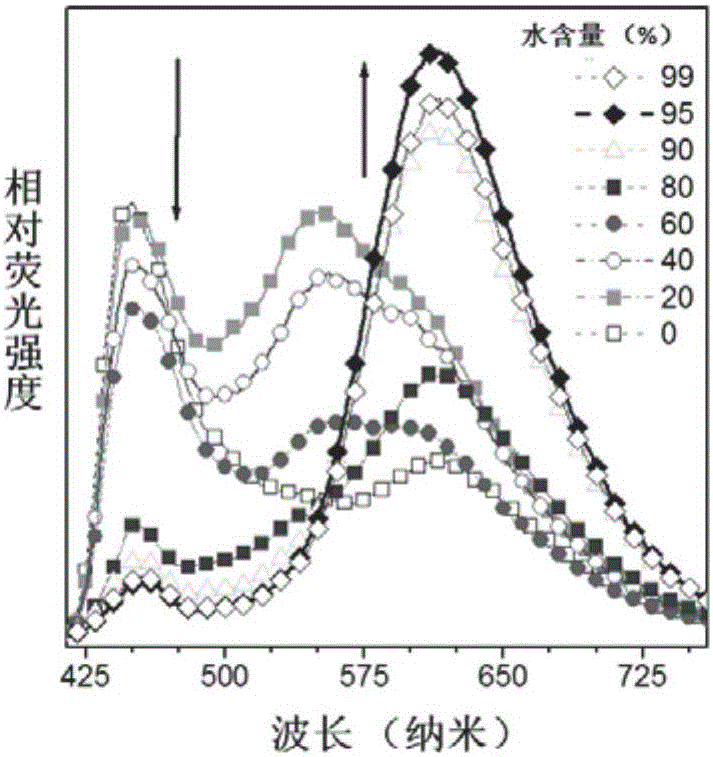
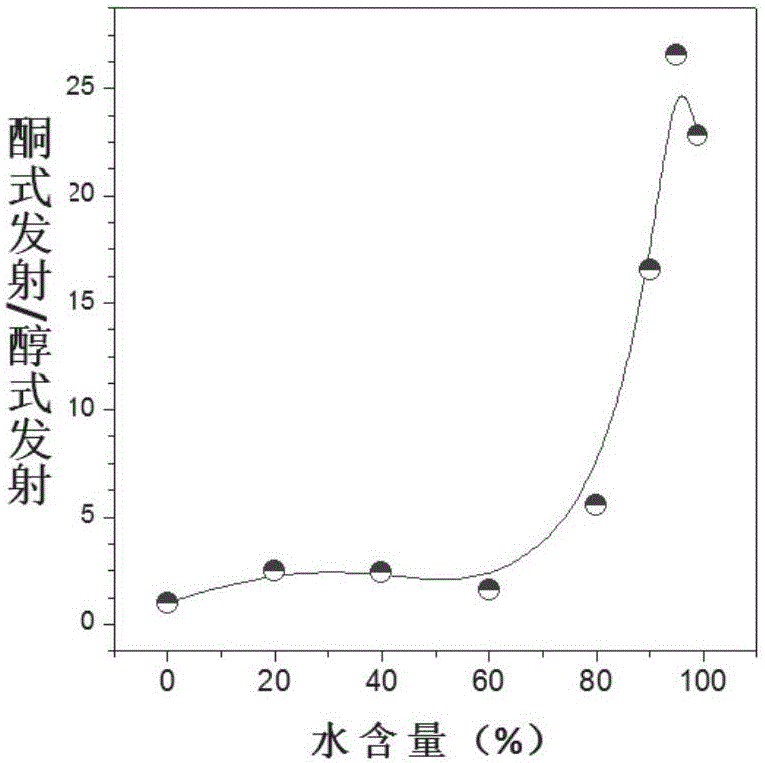
[0083] Example 3: Research on Aggregation-Induced Luminescence (AIE) Properties of TPE-DMNS
[0084] For ESIPT molecules, the aggregation state is mostly ketone emission, so the aggregation luminescence phenomenon at this place was detected. Such as Figure 2A and 2B As shown, a certain proportion of water is continuously added to the tetrahydrofuran solvent of TPE-DMNS (dissolved single molecular state), and TPE-DMNS slowly aggregates into nanoparticles due to solubility problems, and the fluorescence intensity of the alcohol form gradually decreases, and the intensity of the ketone form increases. The proportion of those increases with the increase of water content, indicating that TPE-DMNS has AIE properties. Compared with the Salen-type Schiff base structure of other types of maleonitrile, TPE-DMNS exhibits ESIPT and AIE properties and has the potential of a fluorescent probe.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com