In-vitro IAK immune cell culture method
A culture method and technology of immune cells, applied in the field of tumor prevention and treatment, can solve the problems of limited ability to kill tumor cells and low proportion of NKT cells, and achieve the effect of improving cell killing ability and good tumor killing effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
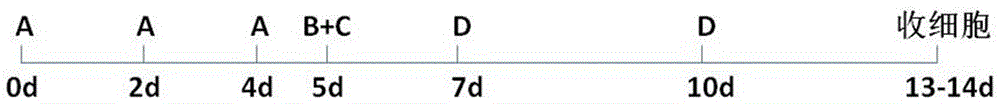
[0055] Using the in vitro IAK immune cell culture method provided by the present invention, the inventor first carried out a small sample experiment, and the specific experimental process is as follows: figure 1 As shown, the details are as follows.
[0056] (1) Extract mononuclear cells, specifically:
[0057] Collect 5 mL of peripheral blood from tumor patients; centrifuge at 1500 rpm / min for 10 min, and store the supernatant at -20°C after inactivation;
[0058] The precipitated cells were resuspended in 30 mL of normal saline, placed on 15 mL of lymphocyte separation medium, subjected to density gradient centrifugation, and mononuclear cells were collected and washed three times with normal saline;
[0059] The density gradient centrifugation was performed at 2500 rpm / min for 25 min.
[0060] (2) In vitro cell culture, the culture process is as follows: figure 1 As shown, specifically:
[0061] Day 0: The peripheral blood mononuclear cells obtained in step (1) were pla...
Embodiment 2
[0080] On the basis of the small sample in Example 1, the inventor further carried out a large sample experiment. The specific process is as follows:
[0081] (1) Extract peripheral blood mononuclear cells, specifically:
[0082] Collect 60 mL of peripheral blood from tumor patients; centrifuge at 1500 rpm / min for 10 min, and store the supernatant at -20°C after inactivation;
[0083] The precipitated cells were diluted to 50 mL with normal saline, placed on four 15 mL lymphocyte separation medium respectively, subjected to density gradient centrifugation, and mononuclear cells were collected and washed three times with normal saline;
[0084] The density gradient centrifugation was performed at 2500 rpm / min for 25 min.
[0085] (2) In vitro cell culture, specifically:
[0086] The lymphocytes extracted in the above (1) were divided into two parts, one part was cultured with IAK cells, and the culture process was the same as in Example 1; the other part was cultured with tr...
Embodiment 3
[0092] The specific process of the in vitro IAK immune cell culture method provided in this example is the same as that in Example 1, except that the amount of 7DW8-5 in reagent B has been appropriately adjusted, and the specific experimental conditions are as follows.
[0093] (1) Extract mononuclear cells, as described in Example 1.
[0094] (2) In vitro cell culture, specifically:
[0095] Divide the lymphocytes extracted in the above (1) into three parts:
[0096] One of them was cultured with IAK cells, and the culture process was the same as in Example 1 (named IAK1);
[0097] When one of them was cultured with IAK cells (named IAK2), the culture procedure was the same as that in Example 1, but the amount of reagent B added was halved, that is, the amount of reagent B added was 5 μL / mL;
[0098] The last part was cultured with traditional CIK cells, and the culture process was the same as that described in Example 1.
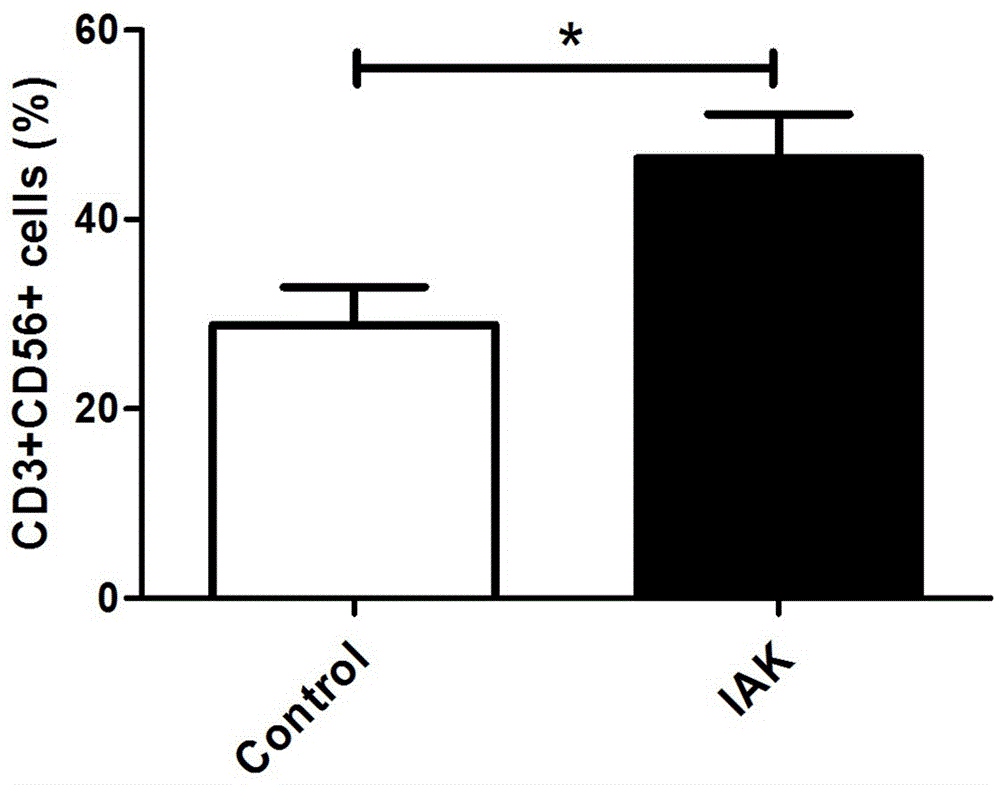
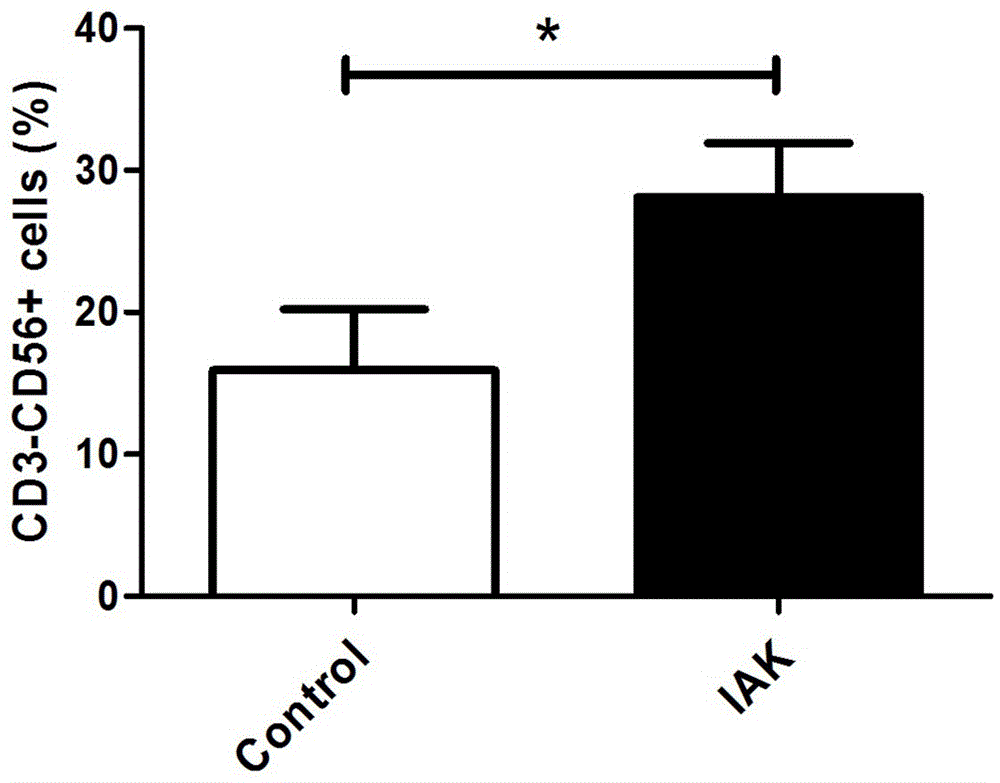
[0099] Finally, the cells are collected, and the im...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com