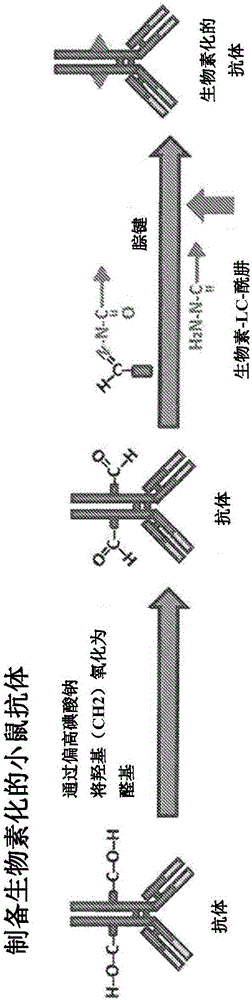
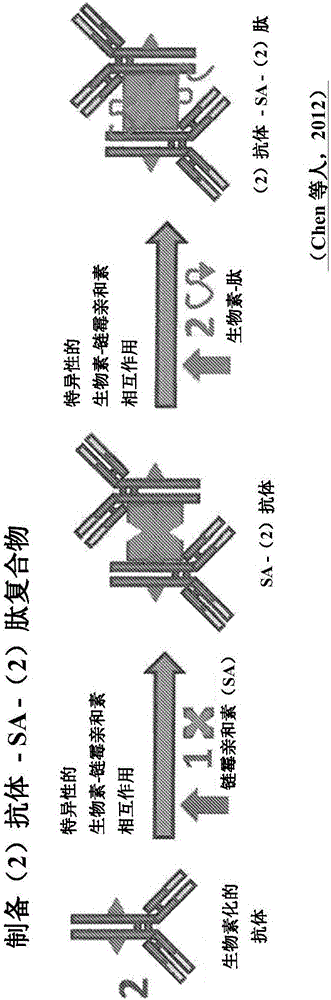
Antibody guided vaccines and methods of use for generation of rapid mature immune responses
An antibody and vaccine technology, applied in the direction of anti-animal/human immunoglobulin, antibody medical components, chemical instruments and methods, etc., can solve the problems of antigen consumption and time-consuming
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
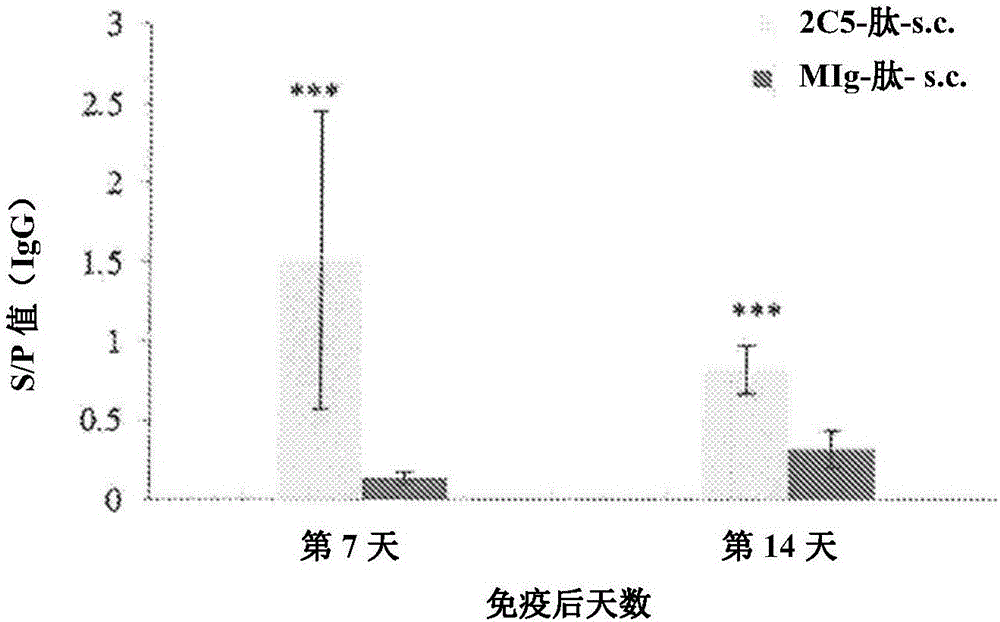
[0071] Example 1: Production and use of chicken CD40 antibodies to induce IgA against the peptide
[0072] Materials and methods
[0073] Anti-cCD40 monoclonal antibody (designated 2C5)
[0074] Our laboratory has previously reported the development of an agonistic anti-cCD40 Mab designated 2C5 (Chen et al., 2010b Development and Comparative Immunology 34: 1139-1143). Mab 2C5 targets recombinant cCD40 extracellular domain (cCD40 ED ) (recombinant cCD40 obtained from CVM-VTPB). This Mab recognizes and binds CD40 as expressed on primary chicken B cells and macrophages, DT40B cells and HD11 macrophages. Mab 2C5 also induced NO production in HD11 macrophages and stimulated DT40B cell proliferation (Chen et al., 2010b). These results demonstrate that 2C5 induces downstream CD40 signaling upon binding CD40 and is thus agonistic. Mab 2C5 at least partially mimics the function of the chicken's natural CD40 ligand, CD154. Chen et al. (2012, J Immunol Methods 378:116-120) also rep...
Embodiment 2
[0095] Example 2: Production of anti-chicken CD40 scFv
[0096] A single-chain antibody library (scFv) against chicken CD40 (chCD40) was constructed by phage display. Briefly, mice were immunized with chicken CD40, and splenocytes were collected. Extract RNA and synthesize cDNA. The variable light and heavy chains were amplified using PCR, and the scFv was amplified using PCR. The product was ligated into a vector and transformed into E. coli. Following rescue of the helper phage, the phage were precipitated. Get 3×10 6 transformant-sized scFv library. The phage library was added to the CD40-coated ELISA, allowed to bind and washed to remove non-specifically bound phage. E. coli was added to allow expansion of bound phage, and the process was repeated. Three rounds of panning against chicken CD40 resulted in a 40% enrichment of positive clones, those predominating in the library are shown in Table 1 below.
[0097] Table 1: Panning to enrich for anti-CD40 scFv
[0098...
Embodiment 3
[0103] Embodiment 3: Avian influenza adjuvant compound test
[0104] Materials and Methods
[0105] Raise monoclonal antibodies against the conserved M2e ion channel domain of AIV. Based on a previously published sequence, the M2e conserved peptide sequence CEVETPTRN (SEQ ID NO: 58) was synthesized and used to immunize Balb / c mice subcutaneously at 50 μg / mouse in RIBI buffer. Three boosts of 25 μg / mouse subcutaneously were performed at three-week intervals. Plasma was collected 1 week after each immunization to screen for peptide-specific IgG responses based on ELISA. Once the mice were hyper-immunized (antibody titers stabilized at plateau), the mice were euthanized and splenocytes were harvested.
[0106] Splenocytes were used for electrofusion with mouse Sp2 / 0 myeloma cells to generate B cell hybridomas. Maintain hybridoma cultures at 37 °C, 5% CO 2 , and cultured in DMEM supplemented with 15% FBS. Hybridoma supernatants were screened by ELISA for the production of pe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com