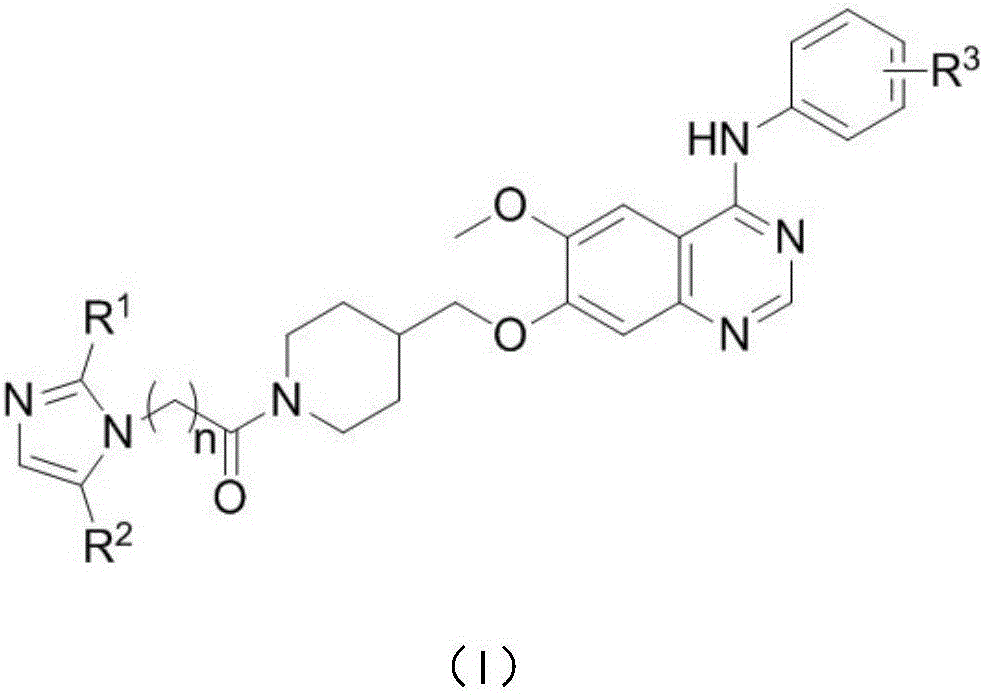
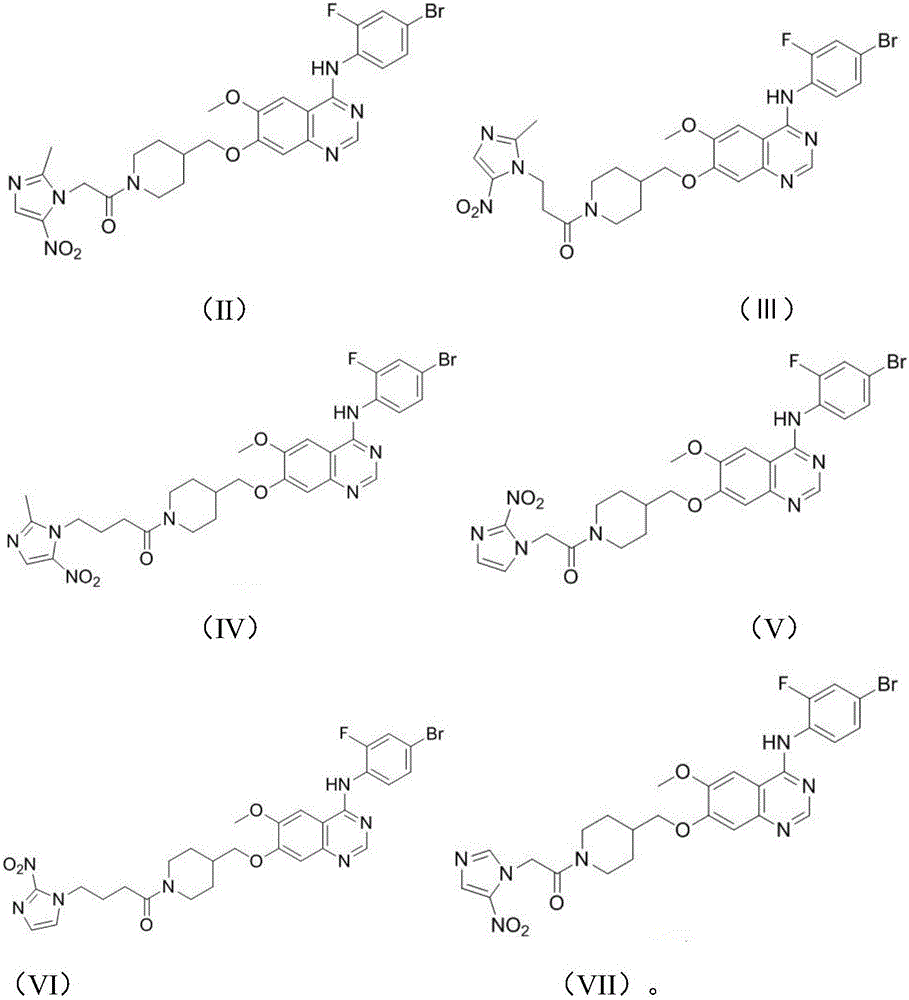
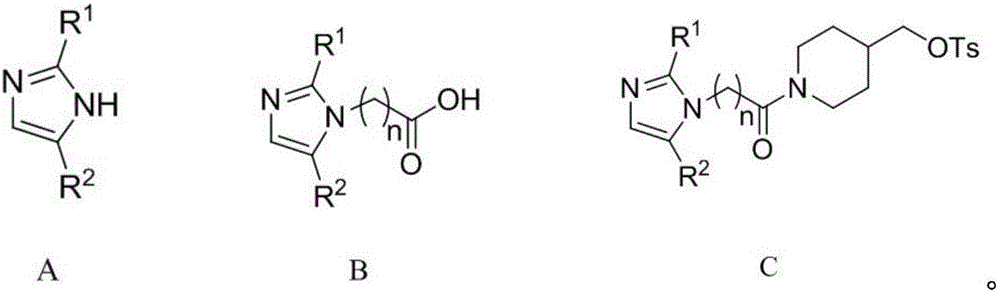
Anilinoquinazoline compound containing nitroimidazole group and preparation method and application thereof
A compound and nitro technology, applied in the field of aniline quinazoline compounds and their preparation, can solve the problems of insignificant curative effect, lack of similar compounds and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
[0060] Example 1 Preparation of 4-hydroxymethylpiperidine-1-carboxylate tert-butyl ester
[0061] Synthesized with reference to the literature J.Med.Chem., 2002, 45, 1300. White solid; melting point 74.3-76.1°C; yield 93.8%. MS M / z: 214.77 [M-1]; 1 H NMR (400MHz, DMSO-d 6 )δ0.95(ddd, J 1 =24.8Hz, J 2 =12.8Hz, J 3 =4.4Hz, 2H), 1.37(s, 9H), 1.49(m, 1H), 1.59(d, J=13.2Hz, 2H), 2.65(s, 2H), 3.23(t, J=6.0Hz, 2H) ), 3.93 (d, J=12.4 Hz, 2H), 4.42 (t, J=5.2 Hz, 1H).
Embodiment 24
[0062] Example 2 Preparation of 4-methylbenzenesulfonic acid-(1-tert-butoxycarbonylpiperidin-4-yl)methyl ester
[0063] Synthesized with reference to the literature J.Med.Chem., 2002, 45, 1300. White solid; melting point 72.4-73.6°C; yield 85.2%. MS M / z: 370.18[M+1]; 1 H NMR (400MHz, DMSO-d 6 )δ0.96(ddd, J 1 =24.4Hz, J 2 =12.4Hz, J 3 =4.4Hz, 2H), 1.36(s, 9H), 1.53(d, J=11.2Hz, 2H), 1.76(m, 1H), 2.41(s, 3H), 2.63(s, 2H), 3.87(d , 4H), 7.47(d, J=8.0Hz, 2H), 7.77(d, J=8.0Hz, 2H).
Embodiment 34
[0064] Example 3 Preparation of 4-p-toluenesulfonyloxymethylpiperidine methanesulfonate
[0065] Synthesized with reference to J.Org.Chem.2010, 75, 8117. White solid; melting point 113.0-114.5°C; yield 84.3%. MS M / z: 270.38[M+1]; 1 H NMR (400MHz, DMSO-d 6)δ1.31(m, 2H), 1.72(d, J=12.8Hz, 2H), 1.93(m, 1H), 2, 32(s, 3H), 2.42(s, 3H), 2.83(m, 2H) ), 3.23(d, J=12.8Hz, 2H), 3.92(d, J=6.0Hz, 2H), 7.48(d, J=8.4Hz, 2H), 7.78(d, J=8.4Hz, 2H), 8.12(br s, 1H), 8.50(br s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com