Gadobutrol injection and preparation method thereof
A technology for gadobutrol and injection, which is applied in the field of gadobutrol injection and its preparation, can solve the problems of strong irritation, many side effects, and high content of free gadolinium, and achieve the effect of less irritation and less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A preparation method of gadobutrol injection, comprising the steps of:
[0040] (1) Add 70% of the prescription amount of fresh water for injection into the preparation equipment, add 0.537 g of disodium hydrogen phosphate, stir and dissolve at a controlled temperature of 40-50 ° C, and obtain the mixed solution A;
[0041] (2) Add 0.513 g of Calcium Sodium Cobutrol and 604.720 g of Gadobutrol to Mixed Solution A, stir and dissolve at a controlled temperature of 40-50° C. to obtain Mixed Solution B;
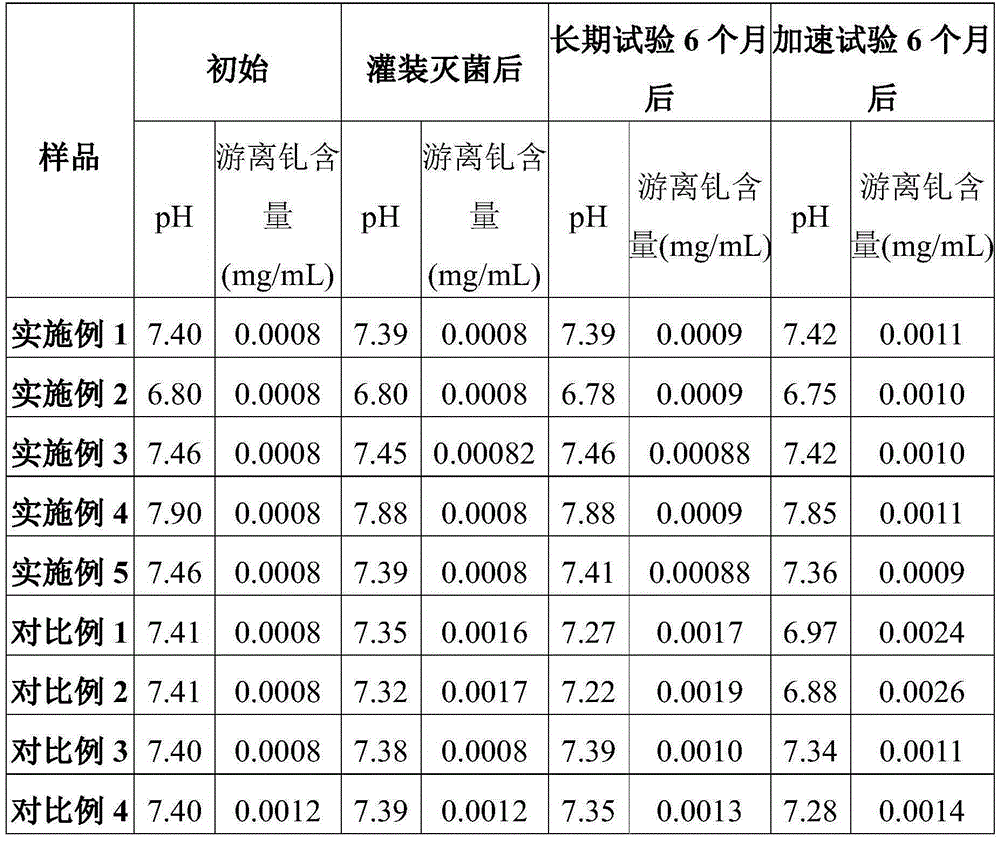
[0042] (3) Dilute the mixed solution B with the remaining prescription amount of water, set the volume to 1000mL, measure the pH value to 7.40, and obtain gadobutrol injection, and determine the content of free gadolinium in the injection to be 0.0008mg / mL.
Embodiment 2
[0044] A preparation method of gadobutrol injection, comprising the steps of:
[0045] (1) Add fresh water for injection of 75% of the prescription amount into the preparation equipment, add 0.358 g of disodium hydrogen phosphate, stir and dissolve at a controlled temperature of 40-50 ° C, and obtain the mixed solution A;
[0046] (2) Add 0.514 g of Calcium Sodium Cobutrol and 604.720 g of Gadobutrol to Mixed Solution A, stir and dissolve at a controlled temperature of 40-50° C. to obtain Mixed Solution B;
[0047] (3) Dilute the mixed solution B with the remaining prescription amount of water, set the volume to 1000mL, and measure the pH value to 6.80 to obtain gadobutrol injection. The free gadolinium content in the injection was determined to be 0.0008mg / mL.
Embodiment 3
[0049] A preparation method of gadobutrol injection, comprising the steps of:
[0050] (1) Add 70% of the prescription amount of fresh water for injection into the preparation equipment, add 0.537 g of disodium hydrogen phosphate, stir and dissolve at a controlled temperature of 40-50 ° C, and obtain the mixed solution A;
[0051] (2) Add 0.515 g of Calcium Sodium Cobutrol and 604.721 g of Gadobutrol to Mixed Solution A, stir and dissolve at a controlled temperature of 40-50° C. to obtain Mixed Solution B;
[0052] (3) Dilute the mixed solution B with the remaining prescription amount of water, set the volume to 1000mL, measure the pH value to 7.46, and obtain gadobutrol injection, and determine the free gadolinium content in the injection solution to be 0.0008mg / mL.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap