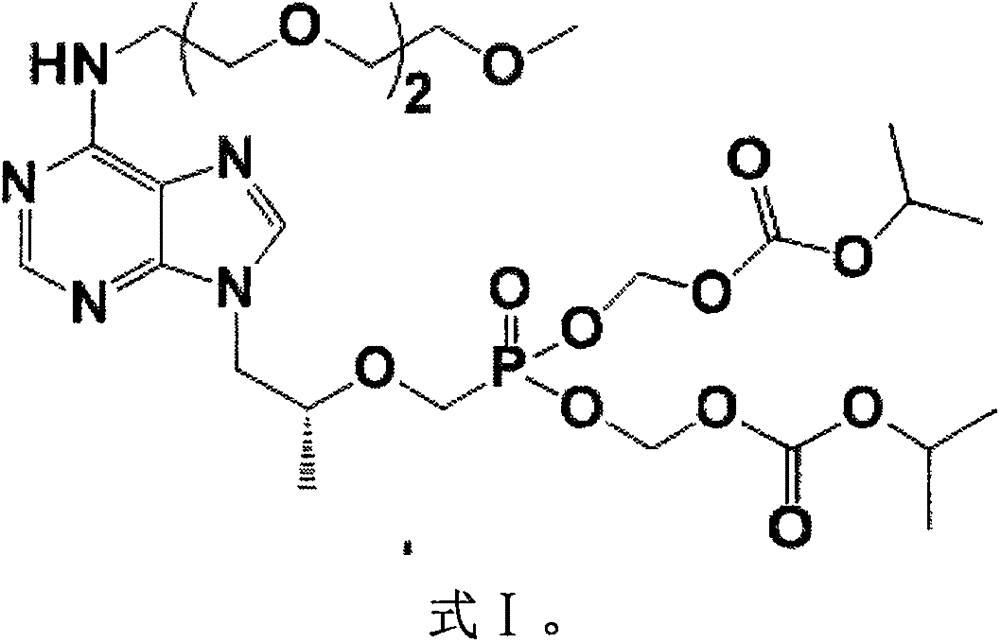
mPEG3-tenofovir disoproxil and preparation method thereof
A tenofovir disoproxil and structural formula technology, applied in the field of preparation of mPEG3- tenofovir disoproxil, can solve the problems that the solvent cannot be recycled, the difficulty of diesterization is increased, and the product quality is difficult to guarantee, so as to achieve good application development Prospects, stable product quality, and the effect of solving solvent loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
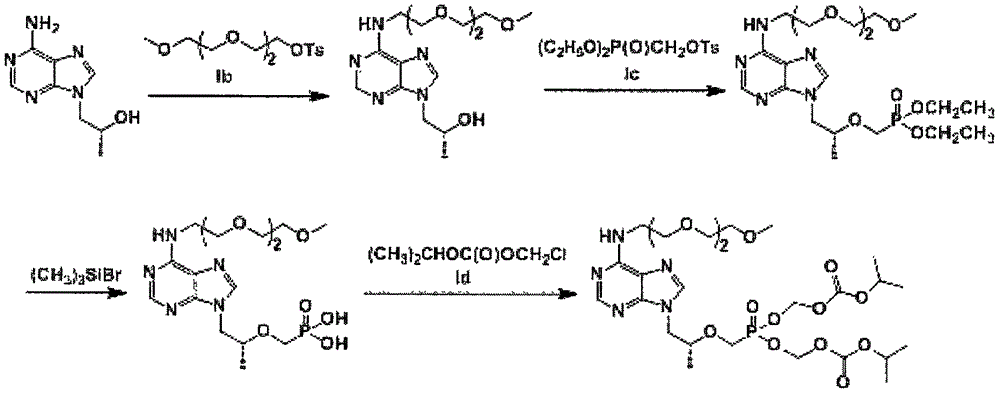
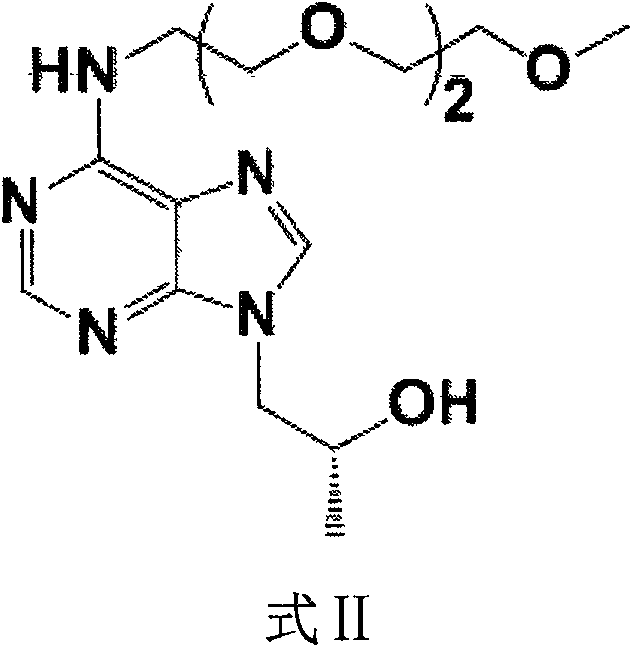
[0053] Example 1 mPEG represented by formula II 3 Preparation of -(R)-9-(2-hydroxypropyl)adenine
[0054] (R)-9-(2-hydroxypropyl) adenine (Ia, 22.8g, 117mmol), triethylene glycol monomethyl ether p-toluenesulfonate (Ib, 67.2g, 211mmol) and K 2 CO 3 (32.4g, 234mmol) was dissolved in DMF (900mL), and heated to 100°C for 7h. After the reaction was completed, cool to room temperature, add distilled water (1200 mL), stir and mix well. Extracted with ethyl acetate (720mL×4), the mixed organic layer was dried over anhydrous sodium sulfate, filtered and concentrated, and the residue was separated by silica gel column to obtain 19.6g of an oily solid product with a yield of 49.4% and a HPLC purity of 98.0%.
[0055]
Embodiment 2
[0056] Example 2 mPEG represented by formula III 3 - the preparation of tenofovir diethyl ester
[0057] mPEG 3 -(R)-9-(2-Hydroxypropyl)adenine (Formula II, 25.4g, 74.8mmol) was dissolved in DMF (150mL), and 2.0M tert-butoxylithium (53.9g, 673mmol) was added slowly Tetrahydrofuran solution, and then slowly added p-toluenesulfonyloxymethyl diethyl phosphate (Ic, 44.8g, 89.8mmol), heated to 35°C and stirred for 8h. After the reaction was completed and cooled to 20°C, glacial acetic acid (6.0 mL) was added, volatiles were removed by distillation under reduced pressure, and distilled water (200 mL) was added. use CH 2 Cl 2 The extracts were extracted in portions, and the extracts were combined and dried with anhydrous sodium sulfate. After filtration and concentration, 12.8 g of an oily solid product was obtained, with a yield of 34.9% and an HPLC purity of 95.8%.
[0058]
Embodiment 3
[0059] Example 3 mPEG represented by formula IV 3 - Preparation of tenofovir
[0060] mPEG 3 - tenofovir diethyl ester (formula III, 19.2 g, 28.8 mmol) was dissolved in acetonitrile (250 mL) and bromotrimethylsilane (25 mL), and stirred overnight at room temperature without air. The volatiles were distilled off under reduced pressure, distilled water (500 mL) was added, and the 2 Cl 2 washing. The aqueous phase was adjusted to pH 3.2 with NaOH aqueous solution, cooled and stirred for 6 h. The solid was collected by filtration, washed successively with ice water and acetone, and dried under vacuum to obtain 6.7 g of solid, with a yield of 53.7% and a purity of 98.0% by HPLC.
[0061]
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap