Method for suppressing glucagon secretion of SGLT2 inhibitor
A glucagon and inhibitor technology, which is applied to medical preparations containing active ingredients, pharmaceutical formulations, organic active ingredients, etc., can solve the problem of increased glucagon secretion, unclear mechanism of glucagon secretion, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0074] The following is an illustration of the invention.
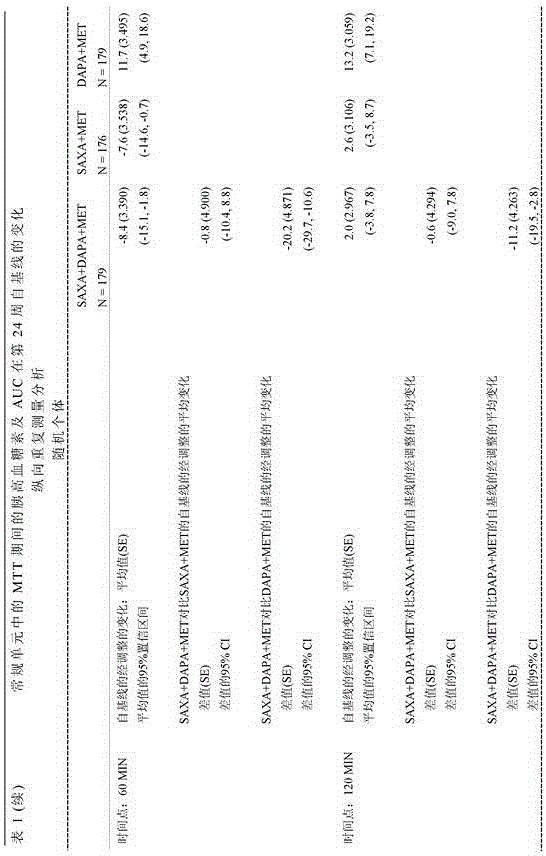
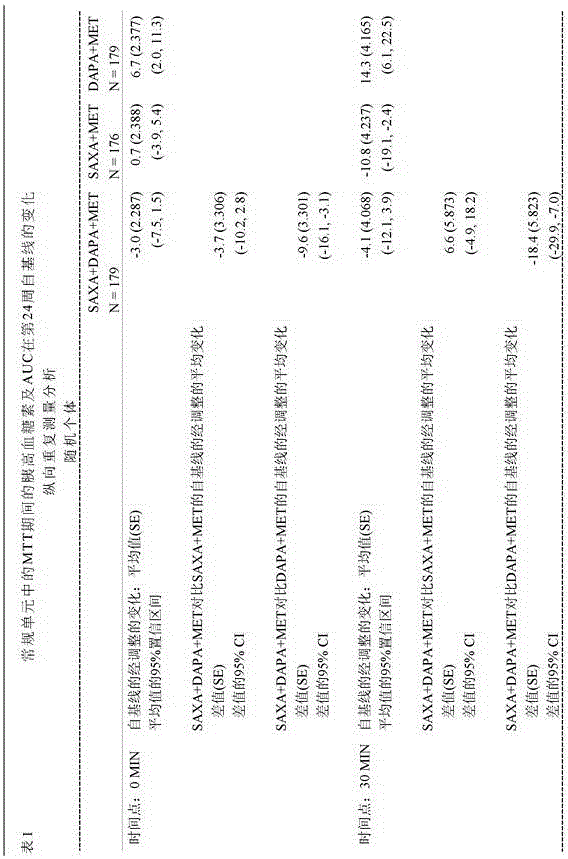
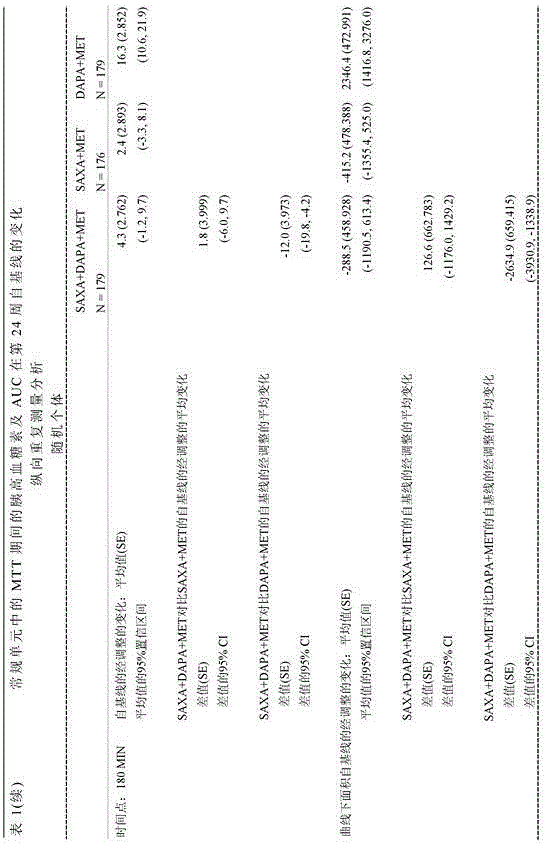
[0075] Adult patients with type 2 diabetes mellitus (T2DM) and HbA1c levels greater than or equal to 8% and less than or equal to 12% who had been on stable metformin therapy for at least 8 weeks at screening were randomized 1:1:1 Receive saxagliptin (5 mg / day) and dapagliflozin (10 mg / day) plus metformin XR (1500-2000 mg / day) dose, saxagliptin (5 mg / day) and placebo plus metformin XR (1500-2000 mg / day) dose, or dapagliflozin (10 mg / day) and placebo plus metformin XR (1500-2000 mg / day) dose for 24 weeks.
[0076] At the beginning of the 4-week run-in period, all patients were switched to the closest metformin XR dose (1,500-2,000 mg / day) during the run-in period and 24 weeks of treatment. Patients were then randomized to receive saxagliptin 5 mg / day and dapagliflozin 10 mg / day plus metformin (SAXA + DAPA + MET), saxagliptin 5 mg / day and placebo plus metformin (SAXA + MET) , or dapagliflozin 10 mg / day and placebo plu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com