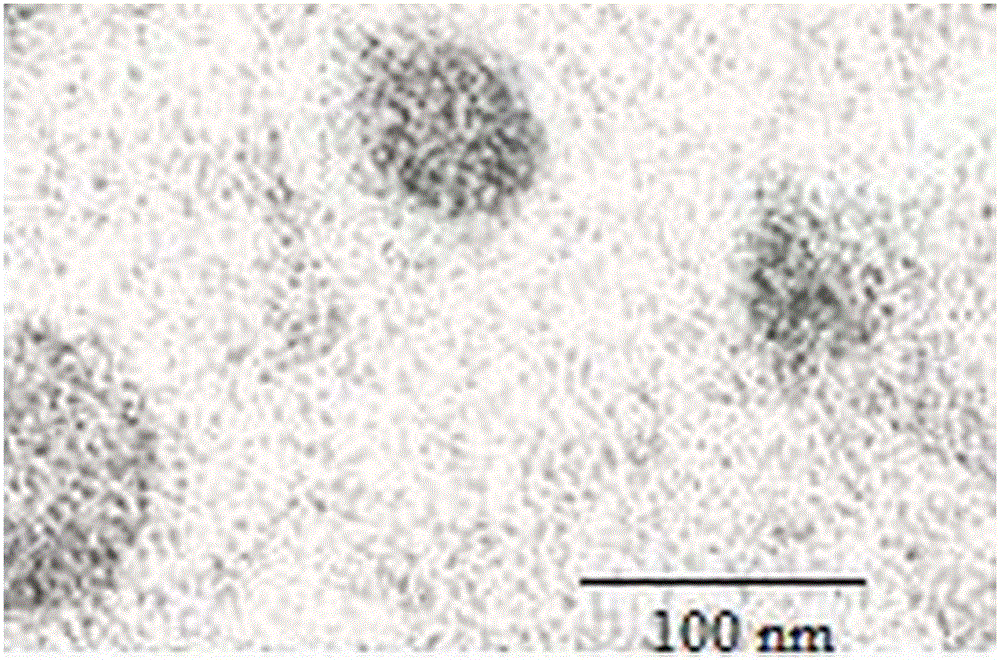
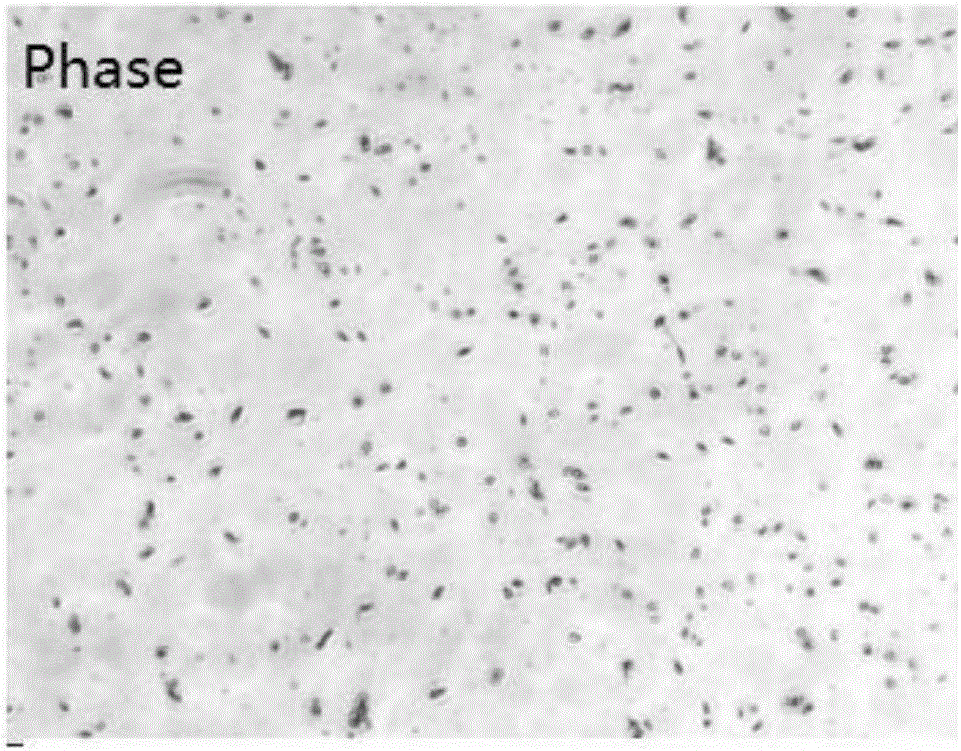
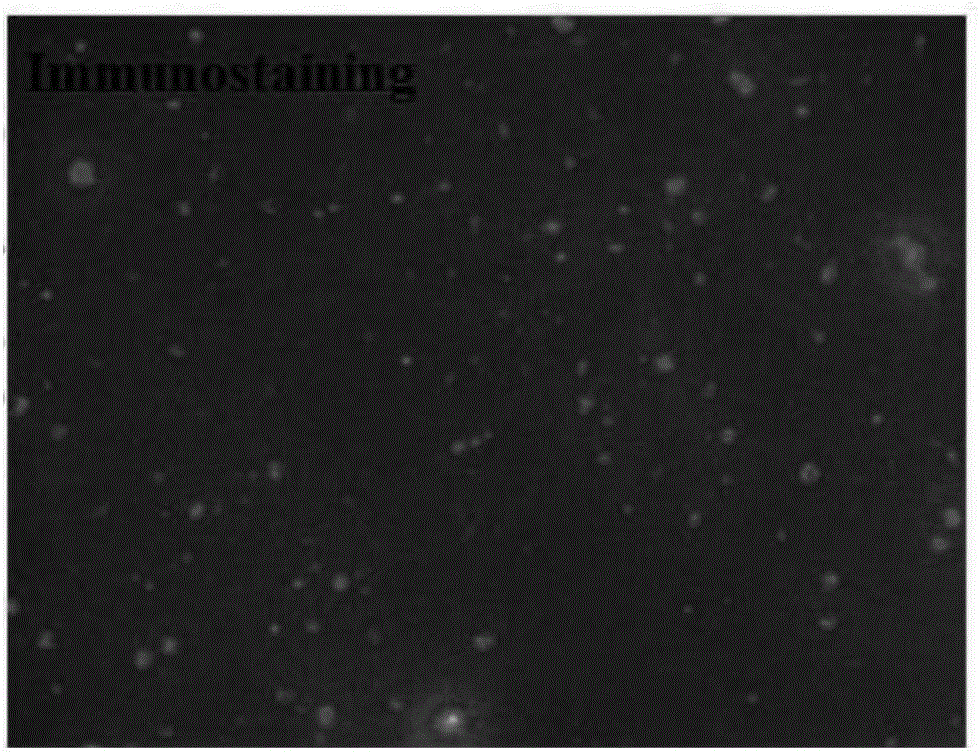
Application of peripheral free exosomes to preparation of liquid-state biopsy tumor diagnosis reagents
A tumor diagnosis and exosome technology, applied in measurement devices, instruments, scientific instruments, etc., can solve the problems that ctDNA detection technology is not suitable for clinical application, the detection effect of early tumor patients is not very good, and the variation of ctDNA quantity is large. , to achieve far-reaching clinical significance and promotion, improve diagnostic specificity, easier access and enrichment effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1 prepares detection kit
[0047] Take 1ml of plasma as the object:
[0048] (1) 200 μl total exosome isolation reagent (Total Exosome Isolation, Invitrogen);
[0049] (2) 20 μl of EpCAM separation magnetic beads (EpCAM beads, Invitrogen), 3 ml of 1×PBS;
[0050] (3) Elisa detection kit, including:
[0051] 100 μl 1:5000 diluted CD9 primary antibody (proteintech) (original concentration 56 μg / 150 μl, concentration after 1:5000 dilution is 0.0000746 μg / μl);
[0052] 100 μl 1:10000 diluted HRP enzyme-labeled secondary antibody (sigma) (original concentration 0.4 μg / μl, concentration after 1:10000 dilution is 0.00004 μg / μl);
[0053] High-binding microtiter plate; 200μl PBST containing 5% skimmed milk as blocking solution; 100μl TMB chromogenic solution; 100μl stop solution; 200μl 1×PBS; 12*200μl 1×PBST.
[0054] Wherein, PBS refers to phosphate buffered saline solution, PBST contains Tween-20 in PBS, and the mass percentage of Tween-20 in the whole PBST sol...
Embodiment 2
[0055] Example 2 Using the kit of Example 1 to detect the expression of CD9 in peripheral free exosomes of patients with renal cancer
[0056] (1) Extraction and purification of tumor exosomes
[0057] ①With the patient’s informed and ethical consent, collect 10ml of preoperative blood samples from tumor patients (pathological biopsy has confirmed renal clear cell carcinoma) in blood collection tubes, centrifuge at 4°C and 300g for 10 minutes, separate To remove the cells in the blood, and then obtain 1ml of the upper layer of plasma.
[0058] ② Centrifuge the plasma at 4°C and 10,000g for 30 minutes, separate to remove organelles and other impurities, and obtain a supernatant.
[0059] ③Put the supernatant into a new centrifuge tube, and add 200 μl of exosome extract (TotalExosome Isolation, Invitrogen) into it to mix, shake evenly, and incubate at 4°C overnight (6-16 hours are acceptable), then, incubate The final mixture was centrifuged at 4° C. and 10,000 g for 1 hour, a...
Embodiment 3
[0097] Example 3 Using the kit of Example 1 to detect the expression of CD9 in peripheral free exosomes of patients with lung cancer
[0098] Using basically the same method as step (1) in Example 2, the collected plasma (1ml) of 30 cases of lung cancer patients and 30 cases of healthy human plasma (1ml) were used to extract and purify tumor exosomes to obtain magnetic beads Bound Epcam-positive (tumor cell-derived) exosome. That is: the plasma sample in (1) is a preoperative plasma sample of a lung cancer patient, and other steps are exactly the same as in Example 2. The method for obtaining the plasma sample is also the same as step (1) ① in Example 2.
[0099] Using the method basically the same as step (2) in Example 2, the ELisa detection of peripheral free tumor exosomes was carried out. The expression of CD9 was detected on the Epcam-positive (tumor cell-derived) exosomes obtained above in Example 3, and analyzed and compared by scatter plots. A scatterplot such as ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com