Preparation method, product and application of a pH-sensitive nano-sized bcl-2 selective inhibitor
A technology of bcl-2 selectivity, applied in medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc., can solve the limitation of clinical treatment of Bcl-2 selective inhibitors, and cannot be too large To maximize the efficacy of the drug, lack of intracellular targeting, etc., to achieve good clinical transformation possibility, good biocompatibility, and the effect of improving drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
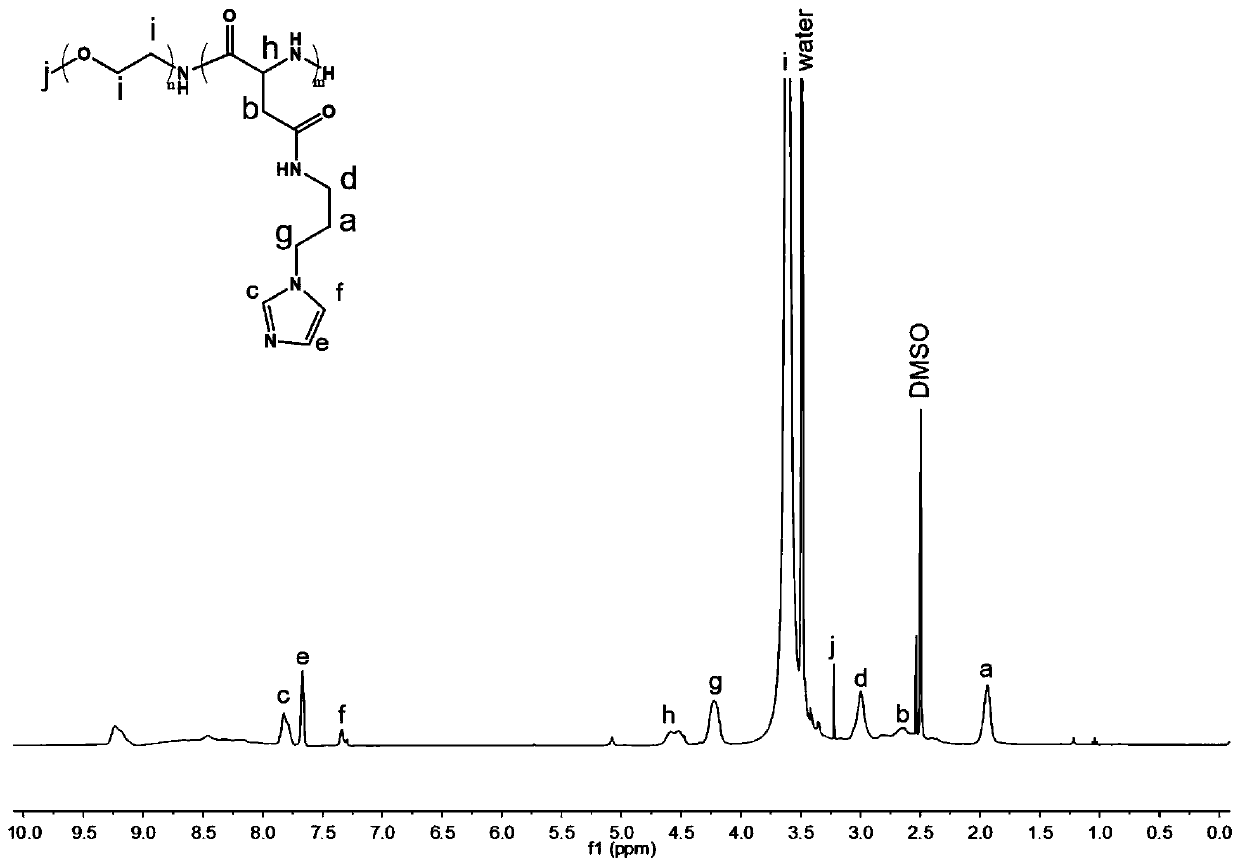
[0040] 1) Synthesis of a pH-sensitive amphiphilic polymer: 0.2 g of ethylene glycol aspartate benzyl ester copolymer was dissolved in 5 ml of N,N-dimethylformamide, and 1 g of 1-( 3-aminopropyl)imidazole. Under the protection of argon, stir at room temperature for 12 hours. After the reaction, the solution was dropped into 0.1N hydrochloric acid solution at 4°C, dialyzed in 0.01N hydrochloric acid solution, and freeze-dried to obtain imidazole-modified ethylene glycol aspartate benzyl ester copolymer. NMR structural characterization of the prepared pH-sensitive amphiphilic polymer, such as figure 1 shown.
[0041] 2) Preparation of a pH-sensitive nano-sized Bcl-2 selective inhibitor: 10 mg of the imidazole-modified ethylene glycol aspartate benzyl ester copolymer obtained in step 1) was dissolved in the Bcl-2 selective inhibitor ABT-1995 mg In 3ml of dimethyl sulfoxide and 1ml of methanol, stirring, rotary evaporation to remove methanol. The solution was then dialyzed for ...
Embodiment 2
[0044]1) Synthesis of a pH-sensitive amphiphilic polymer: 0.2 g of octadecylamine aspartate benzyl ester copolymer was dissolved in 5 ml of dimethyl sulfoxide, and 1 g of 1-(3-aminopropyl base) piperidine. Under the protection of argon, stir at room temperature for 12 hours. After the reaction, the solution was dropped into 0.1N hydrochloric acid solution at 4°C, dialyzed in 0.01N hydrochloric acid solution, and freeze-dried to obtain a piperidine-modified octadecylamineaspartate benzyl ester copolymer.
[0045] 2) Preparation of a pH-sensitive nano-sized Bcl-2 selective inhibitor: the Bcl-2 selective inhibitor ABT-1995mg and the piperidine-modified octadecylamine aspartate benzyl ester copolymer 15mg obtained in step 1) Dissolve in 3ml dimethyl sulfoxide and 1ml methanol, stir, and remove methanol by rotary evaporation. The solution was then dialyzed for two days, and excess polymer was removed by centrifugation, resulting in a pH-sensitive nanosized Bcl-2 selective inhibit...
Embodiment 3
[0047] 1) Synthesis of pH-sensitive amphiphilic polymers: 0.2 g of ethylene glycol benzyl glutamate copolymer was dissolved in 5 ml of dimethyl sulfoxide, and 1 g of 1-(3-aminopropyl ) imidazole. Under the protection of argon, stir at room temperature for 12 hours. After the reaction, the solution was dropped into 0.1N hydrochloric acid solution at 4°C, dialyzed in 0.01N hydrochloric acid solution, and freeze-dried to obtain imidazole-modified ethylene glycol benzyl glutamate copolymer.
[0048] 2) Preparation of a pH-sensitive nano-sized Bcl-2 selective inhibitor: 15 mg of the imidazole-modified ethylene glycol benzyl glutamate copolymer obtained in the Bcl-2 selective inhibitor ABT-1995 mg and step 1) was dissolved in 3ml of dimethylformamide and 1ml of methanol, stirring, rotary evaporation to remove methanol. The solution was then dialyzed for two days, and excess polymer was removed by centrifugation, resulting in a pH-sensitive nanosized Bcl-2 selective inhibitor.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com