Sterilized composition comprising at least one hyaluronic acid and magnesium ascorbyl phosphate
A technology of magnesium ascorbyl phosphate and sterilized composition, applied in the field of sterilized composition, can solve the problems of reduced stability of composition, unimproved stability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
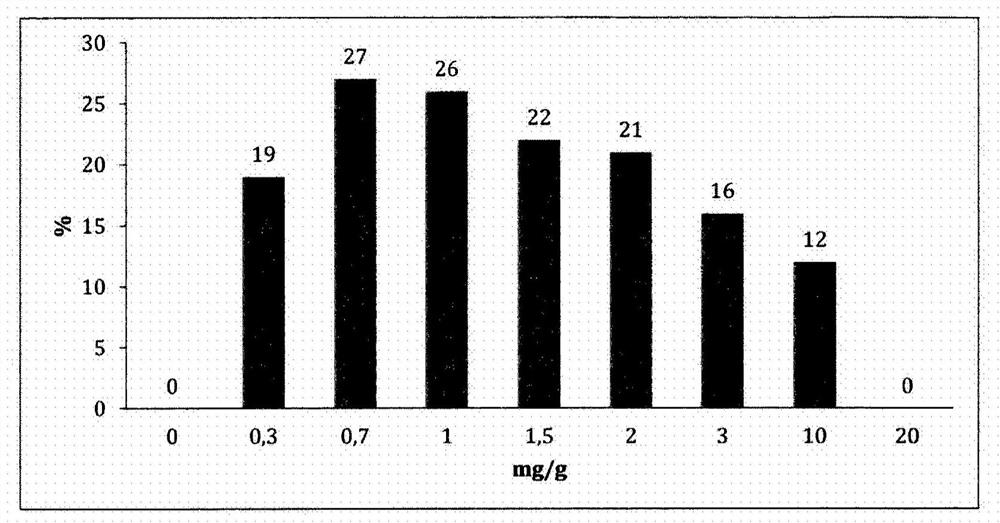
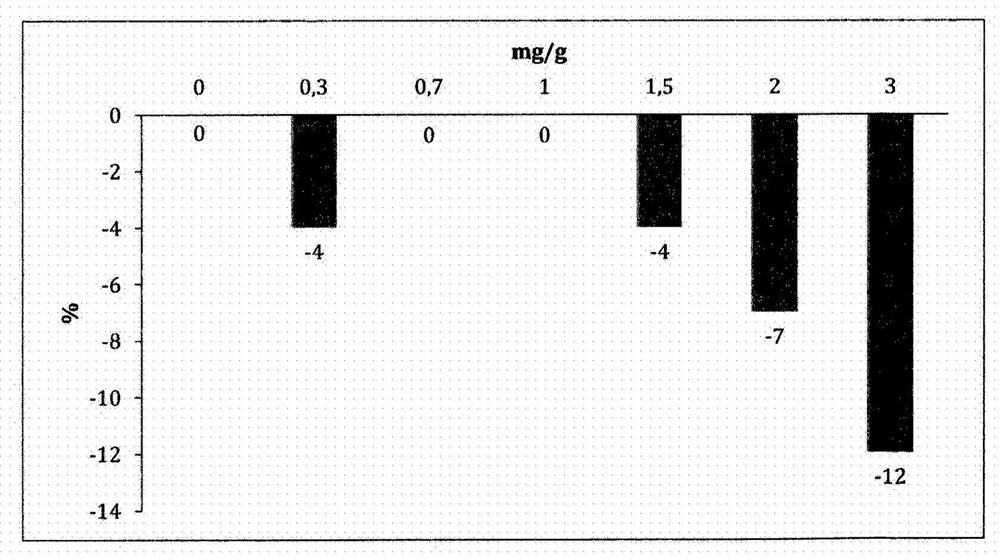
Image
Examples
Embodiment 1
[0183] This example illustrates a composition according to the invention comprising non-crosslinked hyaluronic acid and magnesium ascorbyl phosphate.
[0184] Fibers of injectable grade sodium hyaluronate (NaHA) (1 g; molecular weight: about 2.7 MDa) were weighed into a container. An aqueous solution of phosphate buffer (32.3 g) was added and the whole solution was homogenized using a spatula at room temperature and an atmospheric pressure of 900 mmHg for about 1 hour.
[0185] The NaHA content of the non-crosslinked NaHA hydrogel thus obtained was about 30 mg / g.
[0186] Magnesium ascorbyl phosphate (MAP) (60mg or 2.4×10 -4 mol) was dissolved in phosphate buffer (19.94 g) to obtain an aqueous solution of magnesium ascorbyl phosphate with a content of 3 mg / g.
[0187] The NaHA hydrogel obtained in the above step was diluted by adding a previously prepared aqueous solution of magnesium ascorbyl phosphate. Then, the resulting composition was homogenized.
[0188] This gave a...
Embodiment 2
[0191] This example illustrates an example of a composition according to the invention comprising cross-linked hyaluronic acid and magnesium ascorbyl phosphate.
[0192] Starting with fibers of sodium hyaluronate (NaHA) (1 g; molecular weight: about 2.7 MDa) and butanediol diglycidyl ether (BDDE, 54 mg) by the crosslinking method described in WO 2009 / 071697 by VIVACY (Example 1, Part 1) Obtaining a composition comprising cross-linked hyaluronic acid. The resulting composition contained about 30 mg / g of cross-linked NaHA with a degree of cross-linking X of about 0.12.
[0193] According to Example 1, an aqueous solution of magnesium ascorbyl phosphate with a content of 3 mg / g was prepared.
[0194] The cross-linked NaHA hydrogel obtained in the above step was diluted by adding a previously prepared aqueous solution of magnesium ascorbyl phosphate. Then, the resulting composition was homogenized.
[0195] This gave a composition comprising crosslinked NaHA in an amount of 20 ...
Embodiment 3
[0198] This example illustrates an example of a composition according to the invention comprising cross-linked hyaluronic acid, magnesium ascorbyl phosphate and lidocaine.
[0199] Compositions comprising cross-linked NaHA were prepared by the method described in Example 2, starting with NaHA hydrogel at 30 mg / g and magnesium ascorbyl phosphate at 10 mg / g.
[0200] To the composition obtained above was added a solution of lidocaine at a content of 13 mg / g by the method described in WO 2009 / 024670 by ANTEIS or by the method described in patent application US 61 / 791,977 or FR 13 / 52971 by VIVACY.
[0201] The resulting composition contained cross-linked hyaluronic acid in an amount of 20 mg / g, lidocaine in an amount of 3 mg / g and magnesium ascorbyl phosphate in an amount of 1 mg / g; the mass ratio [HA] / [MAP] was 20.
[0202] The composition thus obtained was packaged in autoclaved (T = 121° C., 10 minutes) syringes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| cross-linking degree | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com