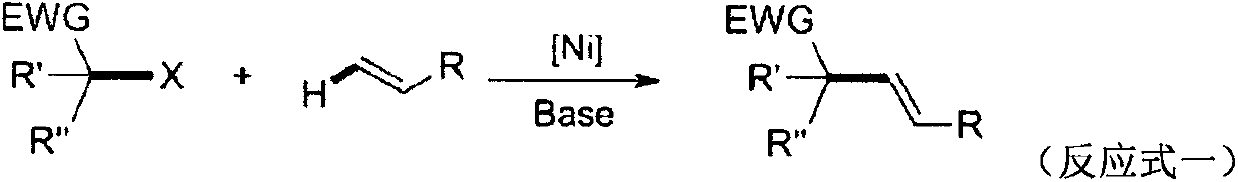
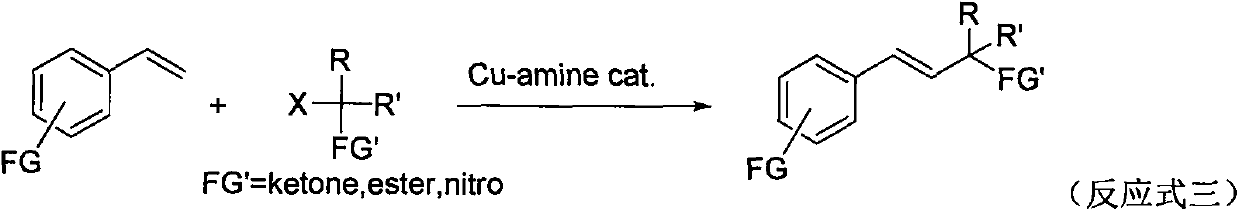
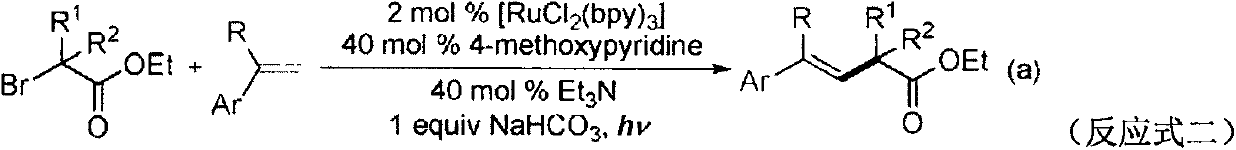A kind of preparation method of β, γ-unsaturated ester compound
An ester compound, unsaturated technology, applied in the beta field, can solve the problems of long reaction time, unsuitable for industrial production and application, etc., and achieves the effects of low price, mild reaction conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Into a dry 10 mL schlenk bottle were added 72.0 mg (0.4 mmol) of 1,1-diphenylethylene, 99.6 mg (0.6 mmol) of methyl 2-bromopropionate, 9.3 mg (0.04 mmol) of silver oxide, and tert-butyl peroxide. Alcohol 72.0 mg (0.8 mmol), triethylamine 80.8 mg (0.8 mmol), N,N-dimethylacetamide (2 mL), and the reaction flask was stirred at 80° C. under argon atmosphere for 24 h. After the completion of the reaction, the reaction solution was washed with saturated brine, extracted with ethyl acetate (3×10 mL), the organic phase was collected, and dried over anhydrous magnesium sulfate. The organic solvent was removed by rotary evaporation, and the residue was separated by silica gel column chromatography (petroleum ether / ethyl acetate) to obtain the target product (methyl 2-methyl-4,4-diphenylbut-3-enoate), yellow Oily liquid, 92% yield. 1 H NMR (400MHz, CDCl 3 ) δ: 7.40-7.32(m, 3H), 7.25-7.19(m, 7H), 6.12(d, J=10.4Hz, 1H), 3.67(s, 3H), 3.32-3.28(m, 1H), 1.27 (d, J=6.8Hz, 3H); 13 C ...
Embodiment 2
[0027] Into a dry 10 mL schlenk bottle were added 72.0 mg (0.4 mmol) of 1,1-diphenylethylene, 99.6 mg (0.6 mmol) of methyl 2-bromopropionate, 11 mg (0.04 mmol) of silver carbonate, and tert-butanol peroxide. 72.0 mg (0.8 mmol), 80.8 mg (0.8 mmol) of triethylamine, N,N-dimethylacetamide (2 mL), and the reaction flask was stirred at 80° C. under an argon atmosphere for 24 h. After the completion of the reaction, the reaction solution was washed with saturated brine, extracted with ethyl acetate (3×10 mL), the organic phase was collected, and dried over anhydrous magnesium sulfate. The organic solvent was removed by rotary evaporation, and the residue was separated by silica gel column chromatography (petroleum ether / ethyl acetate) to obtain the target product (methyl 2-methyl-4,4-diphenylbut-3-enoate) as the product rate 85%.
Embodiment 3
[0029] According to the method of Example 1, the difference is that 117 mg (0.8 mmol) of DTBP is added to replace the butanol in the peroxidation step to obtain the target product in a yield of 15%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com