Novel carbodiimides, method for the production and use thereof
A carbodiimide, carbon atom technology, applied in the field of protection from hydrolysis degradation, can solve the problems of insufficient reactivity stabilization, no, no hydrolysis-inhibition effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0074] Exemplary implementation
[0075] Test on:
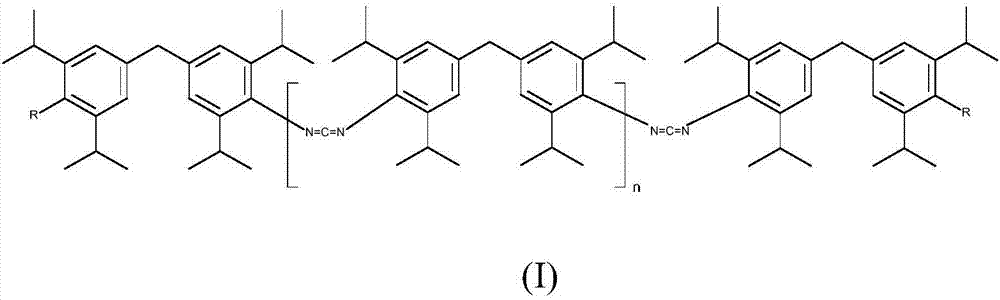
[0076] 1) CDI(A): Carbodiimides according to formula (I), wherein R=NCO and n>20, have an NCN content of about 11 wt % and have an NCO content of <1 wt %, comp.
[0077] 2) CDI(B): carbodiimides of formula (I), wherein R = -NHCOOR III and R III = Cyclohexyl with an NCN content of about 6 wt% and n = about 3, invented.
[0078] Comparative Carbodiimides Production of CDI(A)
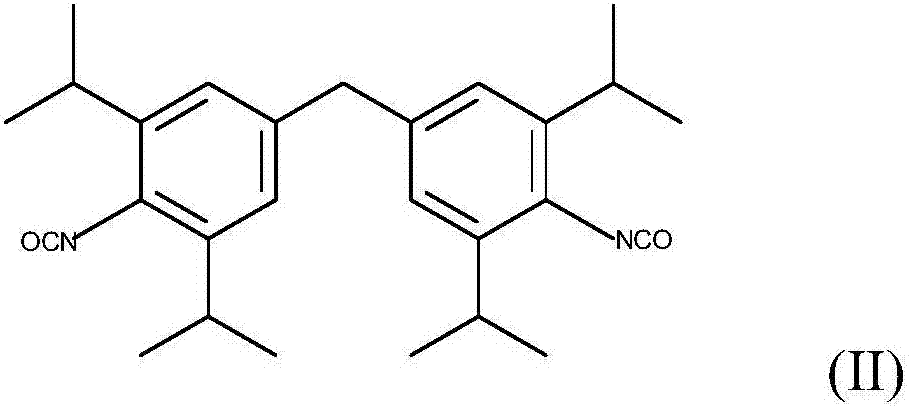
[0079] A 250 ml four-necked flask, which had been baked and purged with nitrogen, was initially filled with 92 g of the diisocyanate of formula (II) (M-DIPI) under a nitrogen stream. 50 mg of 1-methylphosphene oxide were added and the mixture was heated to 160°C. Carbodiimidization (in which carbon dioxide is eliminated) is then carried out at 160° C. until an NCO content of about 1% by weight has been achieved. The product obtained at 160° was no longer stirrable. At 140°C the viscosity is >1000 Pas and thus granulation is not possible.
[008...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com