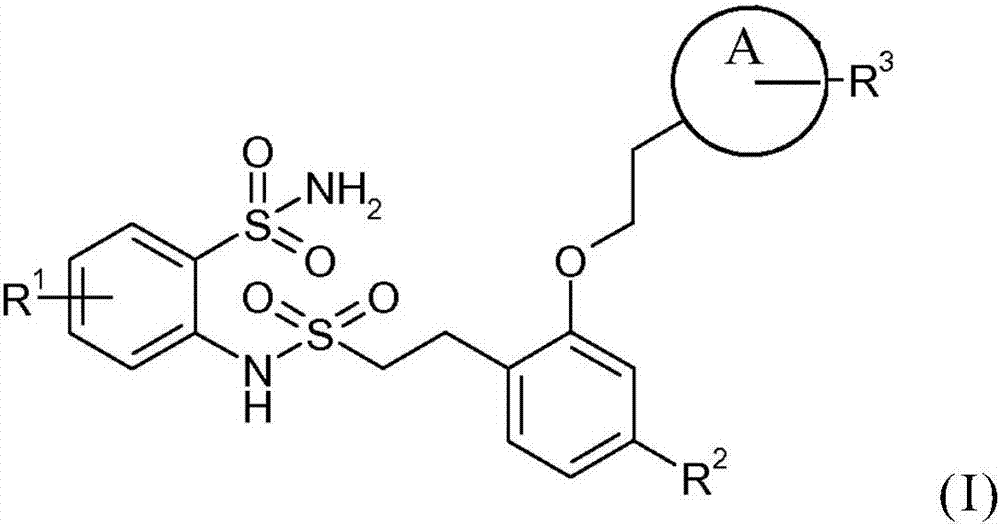
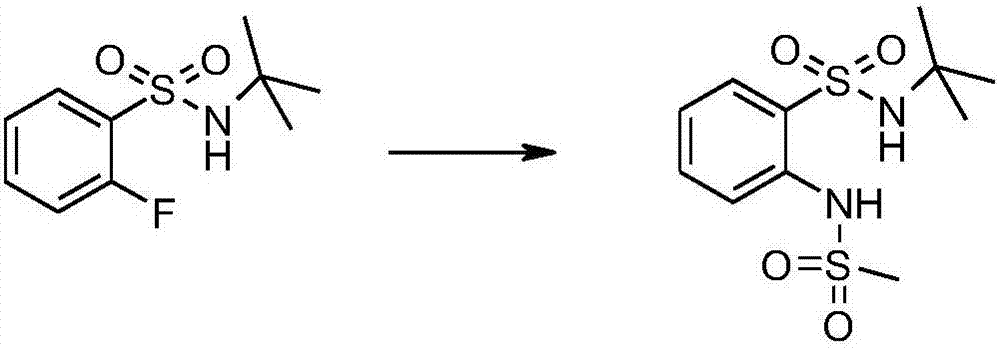
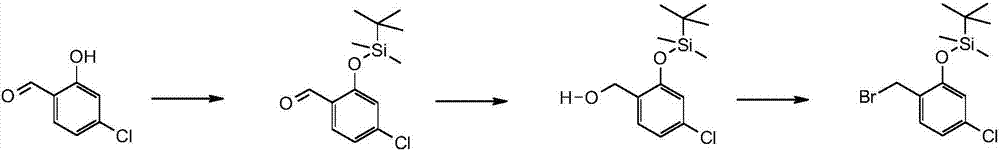
Bis(sulfonamide) derivatives and their use as mpges inhibitors
一种苯磺酰胺、乙基亚磺酰氨基的技术,应用在酰胺有效成分、抗炎剂、药物组合等方向,达到改善效力和选择性、降低副作用、降低胃肠毒性和肾脏毒性的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0129] Compound preparation
[0130] The compounds of the present invention can be prepared as free bases or pharmaceutically acceptable salts thereof by the following methods. In the following description of such methods, it is understood that suitable protecting groups, where appropriate, will be added and subsequently removed from various reactants and intermediates in a manner readily understood by those skilled in the art of organic synthesis. Conventional methods of using such protecting groups as well as examples of suitable protecting groups are eg described in Protective Groups in Organic Synthesis by T.W. Greene, P.G.M Wutz, 3rd Edition, Wiley-Interscience, New York, 1999.
[0131] general method
[0132] All solvents used were of analytical grade, and commercially available anhydrous solvents were routinely used for reactions. Starting materials used were obtained from commercial sources or were prepared according to literature procedures. Room temperature means...
Embodiment 1
[0288] 2-(2-(4-Chloro-2-phenylethoxyphenyl)ethylsulfonamido)benzenesulfonamide
[0289]
[0290] Dissolve N-tert-butyl-2-(2-(4-chloro-2-phenethoxyphenyl)ethylsulfonylamino)benzenesulfonamide (0.058g, 0.11mmol) in 2,2,2 - in trifluoroacetic acid (1.5ml, 19.47mmol) and stirred for 5h. The reaction mixture was co-evaporated with toluene and purified by preparative HPLC to afford the title compound (0.033 g, 64%). 1 H NMR (500MHz, DMSO-d 6 )δppm2.84-2.98(m,4H)3.37-3.46(m,2H)4.13(t,2H)6.88(dd,1H)7.00(d,1H)7.13(d,1H)7.17-7.24(m,1H )7.24-7.36(m,5H)7.55-7.69(m,2H)7.81-7.95(m,3H)8.95(s,1H); MS(ES - )m / z493,495,497[M-H] - .
Embodiment 2
[0292] 2-(2-(4-Chloro-2-(2-methoxyphenethoxy)phenyl)ethylsulfonamido)benzenesulfonamide
[0293]
[0294] To N-tert-butyl-2-(2-(4-chloro-2-(2-methoxyphenethoxy)phenyl)ethylsulfonamido)benzenesulfonamide (77mg, 0.13mmol) Trifluoroacetic acid (1 mL, 13.06 mmol) was added, and the mixture was stirred at room temperature for 2 hours. The solvent was evaporated, followed by co-evaporation with toluene (1 mL). Purification by preparative HPLC afforded the title compound (39.0 mg, 56.1%). 1 HNMR (500MHz, DMSO-d 6 )δppm 2.84-2.94(m,4H)3.43(m,2H)3.81(s,3H)4.05(t,2H)6.83-6.90(m,2H)6.96(d,1H)7.03(d,1H)7.13( d,1H)7.21(m,2H)7.31(t,1H)7.57-7.66(m,2H)7.84(s,2H)7.88(d,1H)8.95(s,1H); MS(ES - )m / z 523,525[M-H] - .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com