Application of substituted cinnamamide derivative to preparation of anxiolytic medicine
一种桂皮酰胺、衍生物的技术,应用在取代桂皮酰胺衍生物在制备抗焦虑药物中的应用领域,能够解决运动性不安、未见到桂皮酰胺衍生物报道、很难静下心来等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
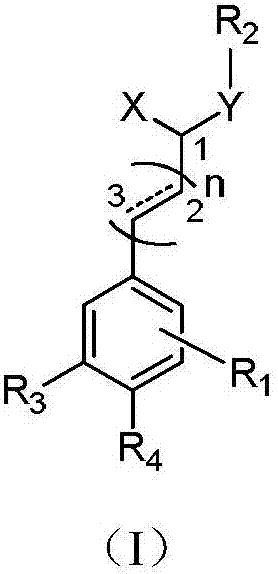
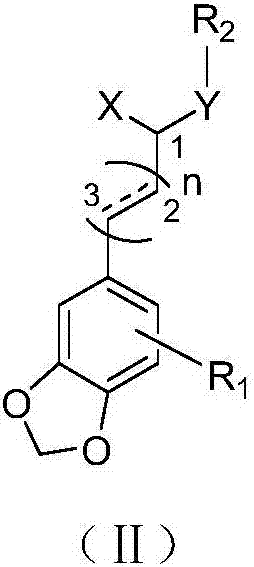
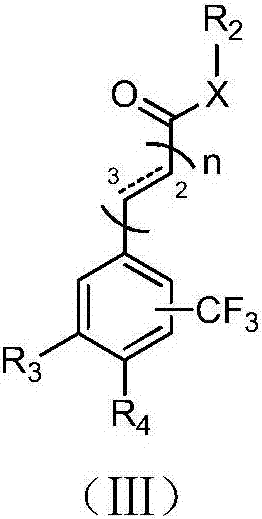
Image
Examples
Embodiment 1
[0099] Example 1: (E)-N-(4-methylpiperazinyl)-5-(5'-trifluoromethyl-3',4'-methylenedioxyphenyl)-1-pentene Amide hydrochloride (Ⅱ-13)
[0100]
[0101] Step 1: Put compound 1 (1.53g, 4.0mmol) in a 100ml single-necked bottle, add 15ml of chloroform to dissolve, then add compound 2 (1g, 4.0mmol) to the reaction system, stir at room temperature for 14h; TLC monitoring ( The developer PE:EA=5:1) showed that the reaction was complete; then the reactant was directly concentrated to obtain the crude product compound 3, and after purification by silica gel column chromatography (PE:EA=5:1), 1.1 g of white solid compound 3 was obtained, Yield 78.87%.
[0102] Step 2: Dissolve compound 3 (0.3g, 0.87mmol) in 5ml of dichloromethane, cool down to 0°C; add trifluoroacetic acid (1ml) dropwise to the reaction system, and then react at room temperature while the reaction system is warming up; After 1 h, TLC showed that the reaction of the raw materials was complete, and the reaction system...
Embodiment 2
[0106] Example 2: (2E,4E)-N-isobutyl-7-(5'-trifluoromethyl-3',4'-methylenedioxyphenyl)-2,4-heptadienamide (Ⅱ-14)
[0107]
[0108] Step 1: Add compound 5 (650mg, 2.6mmol), 20ml anhydrous tetrahydrofuran, lithium hydroxide (326mg, 7.8mmol) into a 50ml three-necked flask, N 2 Heated to 70°C under protection for 1 hour reaction. Compound 1 (0.76g, 2.0mmol) was dissolved in 10ml of anhydrous tetrahydrofuran, and it was dropped into the reaction flask within 0.5 hours. The reaction solution was reacted at 70° C. for 10 hours. TLC detection, stop heating after the reaction is complete. The reaction solution was concentrated to dryness by rotary evaporation, and 20 ml of distilled water was added to dissolve the solid. 2N hydrochloric acid was slowly added dropwise to the above solution until the pH was 2.0, and the stirring was continued for 1 hour. A light yellow solid precipitated, which was collected by suction filtration under reduced pressure and dried in vacuo to obtain...
Embodiment 3
[0112] Example 3: (2E,4E)-N-(4-methylpiperazinyl)-7-(5'-trifluoromethyl-3',4'-methylenedioxyphenyl)-2, 4-Heptadienamide Hydrochloride (Ⅱ-15)
[0113]
[0114] Compound 6 (0.52g, 1.66mmol), N-methylpiperazine (0.5g, 5mmol), DIPEA (0.43g, 3.34mmol) were added to dichloromethane, then HATU (0.95g, 2.5mmol) was added at room temperature Stir for 6h; the reaction system was washed with water, the organic phase was dried and concentrated to obtain a crude product, which was purified by silica gel column chromatography to obtain 350 mg of a colorless oil; then dissolved with 2 ml of dioxane, and then added with 4 ml of dioxane hydrochloric acid solution , stirred at room temperature for 30 min, concentrated and dried to obtain 370 mg of compound II-15 (53%).
[0115] 1 H NMR(MeOD,400MHz):δ7.25(1H,dd,J 1 =10.8Hz,J 2 =4.4Hz),6.96(1H,s),6.87(1H,s),6.48(1H,d,J=14.8Hz),6.31(1H,dd,J 1 =10.8Hz,J 2 =4.4Hz),6.21(1H,m),6.10(2H,s),4.87(2H,br),3.77-3.50(3H,br),3.40-3.10(3H,br),3.15(3H,s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com