Application of gp96 protein to preparation of medicine for treating idiopathic thrombocytopenic purpura
A platelet and idiopathic technology, applied in the field of biomedicine, can solve the problems of high mortality and low morbidity, and achieve the effect of reducing spleen coefficient and increasing the number
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
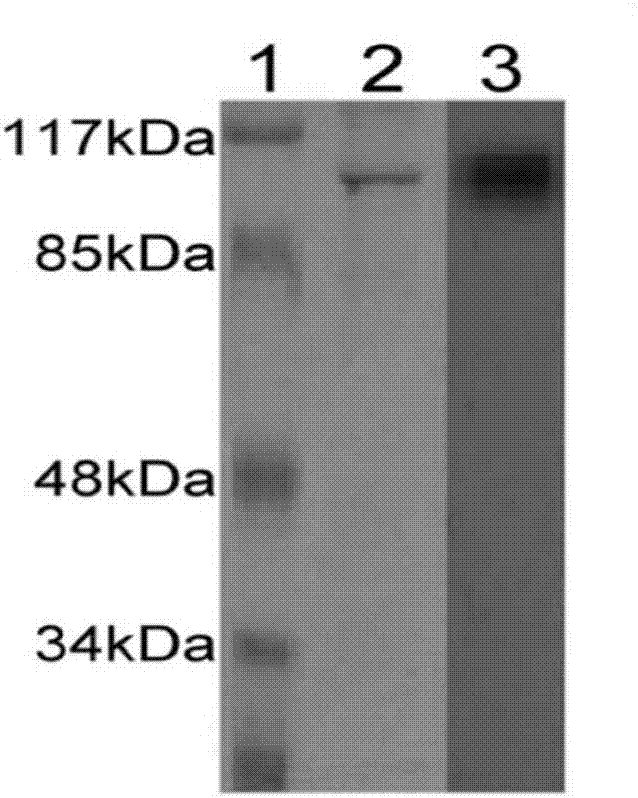
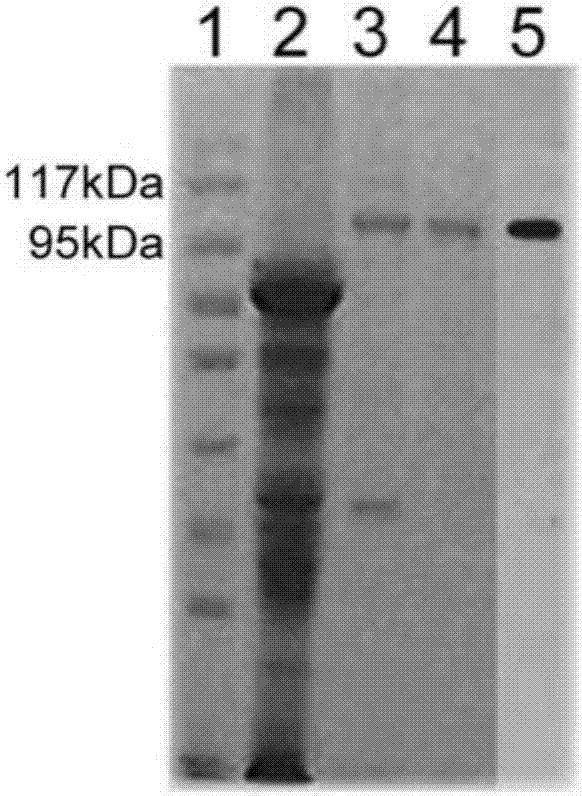
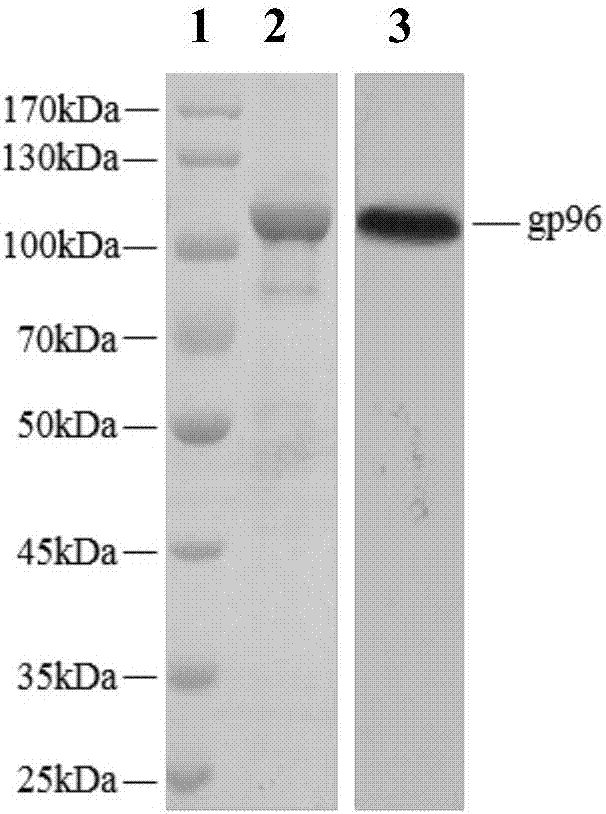
[0043] Example 1 Obtaining of heat shock protein gp96
[0044](1) Purification of gp96 protein from placental tissue
[0045] 1. Tissue homogenate
[0046] Homogenization buffer formula: add PMSF (phenylmethylsulfonyl fluoride, molecular formula C 7 h 7 FO 2 S) to a final concentration of 1 mM (prepared in a beaker on ice: PMSF decomposes in an aqueous solution within a few hours, and the solution cannot be left overnight).
[0047] Take the beaker and place it on ice, quickly cut the placental tissue into fragments with a diameter of about 1-2 mm with scissors in the beaker, and then add homogenization buffer 8 times the weight of the tissue. Stir the tissue pieces into a glass homogenizer, homogenize until the bottom tissue pieces disappear, and then homogenize up and down for more than 15 times. The homogenate was poured into a centrifuge tube, centrifuged at 50,000 g for 60 min, and the supernatant was added to 1 / 10 volume of 10×PBS (pH7.5, 200 mM NaCl) for ConA-Sepha...
Embodiment 2
[0102] Example 2 Determination of heat shock protein gp96 in activated regulatory T cells
[0103] 1. Immunization of mice in groups
[0104] 100 mice weighing 18-22 g were randomly divided into placenta gp96 treatment group 1, placenta gp96 treatment group 2, placenta gp96 treatment group 3, yeast gp96 treatment group 1, gp96 treatment group 2, yeast gp96 treatment group 3, and insect gp96 treatment group Treatment group 1, insect gp96 treatment group 2, insect gp96 treatment group 3 and control group (10 in each group) were processed as follows respectively:
[0105] Placenta gp96 treatment group 1: On the first day of the experiment, the gp96 solution extracted from human placenta tissue in Example 1 was injected intraperitoneally; on the eighth day of the experiment, the gp96 solution extracted from human placenta tissue in Example 1 was injected intraperitoneally again; The gp96 solution extracted from human placenta tissue in Example 1 was injected intraperitoneally aga...
Embodiment 3
[0130] Example 3 Application of gp96 in the treatment of immune thrombocytopenic purpura
[0131] 1. Analysis of platelet count
[0132] 1. Preparation of guinea pig antiplatelet antiserum (APS)
[0133] Take healthy BALB / C mice, take blood from the eye socket, rest for 1-2h, centrifuge at 700rpm / min for 10min, take the upper serum and centrifuge at 1500rpm / min for 15min, take the upper serum and centrifuge at 900rpm / min for 10min, take the upper serum and centrifuge at 3000rpm / min for 10min, Discard the serum, get the precipitated platelets at the bottom, add 1mL of 1% (mass volume fraction) ammonium oxalate, stand still for 5min, centrifuge at 3000rpm / min for 10min, discard the upper liquid, wash the platelets at the bottom three times with normal saline, count, and dilute with normal saline to a concentration of 1 x 10 9 -2×10 9 pc / L, respectively mixed with Freund's complete adjuvant and Freund's incomplete adjuvant at a ratio of 1:1 to form a Freund's complete adjuvant...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com