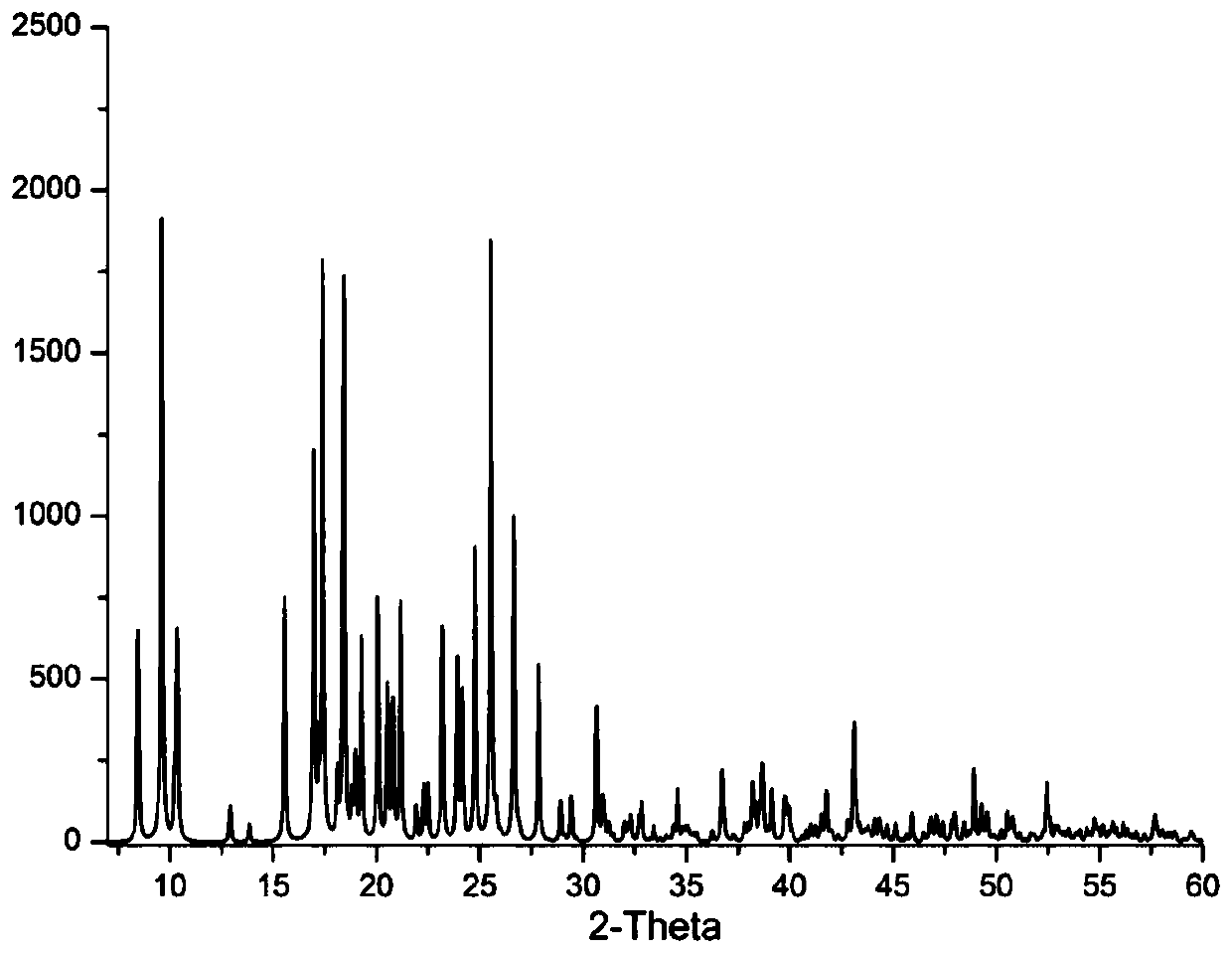
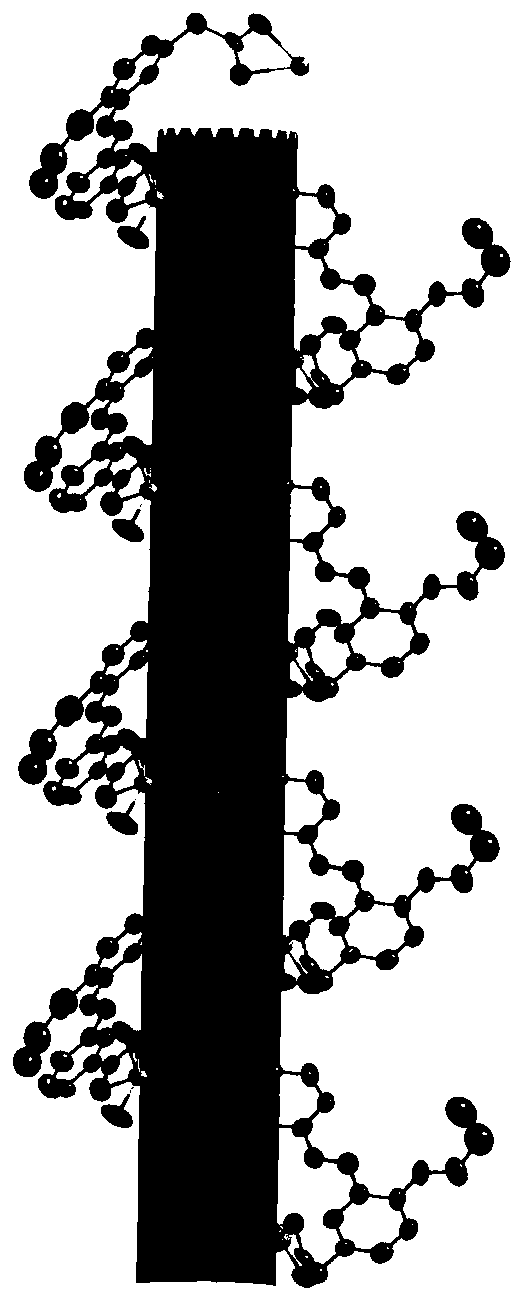
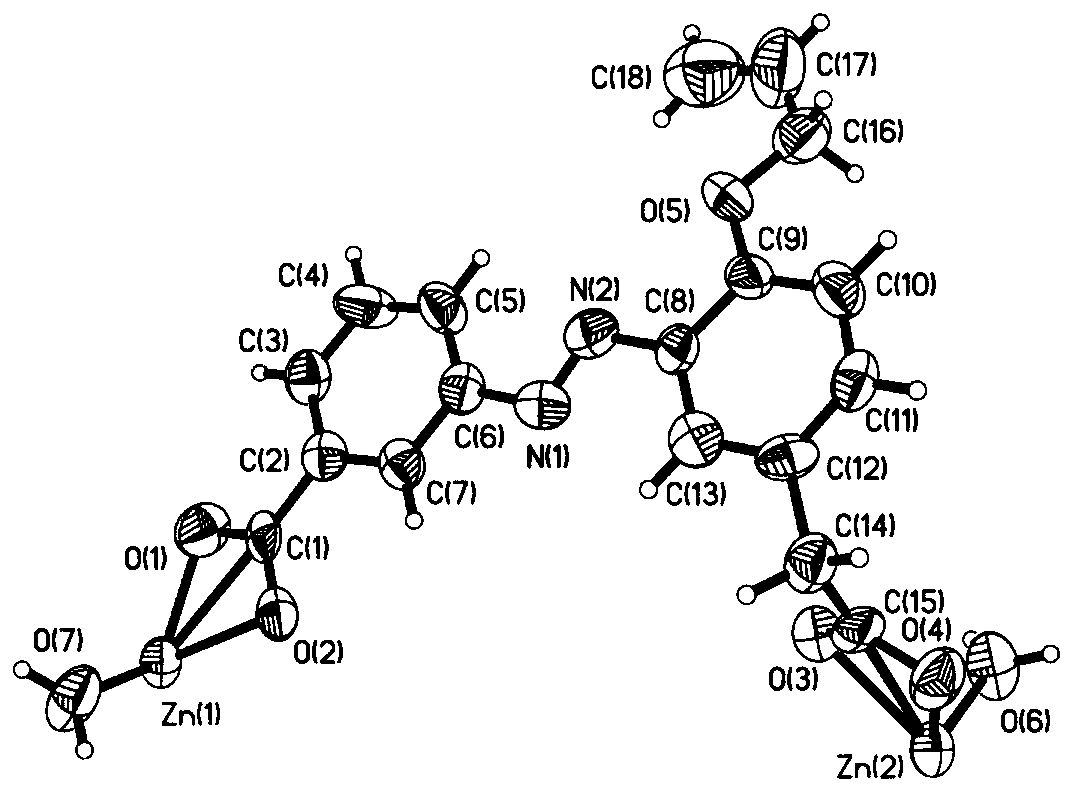
A kind of chiral coordination polymer crystal and preparation method thereof
A technology for coordination polymers and crystals, which is applied in the field of chiral coordination polymer crystals and their preparation, can solve the problem that crystals do not reflect optical rotation properties, and achieves the effects of controllable structure, simple method and low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] In the second aspect, the present invention provides a method for preparing chiral coordination polymer crystals, including the following steps:
[0030] Step A, providing the ligand 3-(3-carboxy)-4-allyloxyazophenylacetic acid;
[0031] In step B, the organic solution of the ligand and the aqueous solution of the Zn salt are respectively configured, the organic solvent and the organic solution of the ligand are sequentially added to the aqueous solution of the Zn salt, and the reaction is allowed to stand still to obtain an orange flake crystal product.
[0032] Preferably, in the step A, the ligand 3-(3-carboxy)-4-allyloxyazophenylacetic acid is synthesized by the Williamson reaction after the diazotization coupling reaction, and the specific preparation steps are as follows:
[0033] Using methyl meta-aminobenzoate as a raw material, diazonium salt is prepared by diazotization reaction with nitrous acid at low temperature, and then reacted with ethyl p-hydroxyphenylacetate to...
Embodiment 1
[0039] Weigh 20 mg of 3-(3-carboxy)-4-allyloxy azophenylacetic acid and dissolve it in 2 mL of ethanol to obtain an ethanol solution of the ligand;
[0040] Weigh 30 mg of zinc nitrate and dissolve it in 2 mL of water to obtain an aqueous solution of zinc nitrate;
[0041] The aqueous solution of zinc nitrate was added to a 5 mL flat-headed test tube, and 1 mL of ethanol was added along the wall, and then the ethanol solution of the ligand was slowly added. After the reaction was allowed to stand for seven days, orange flake crystals were obtained.
Embodiment 2
[0043] Weigh 42 mg of 3-(3-carboxy)-4-allyloxy azophenylacetic acid and dissolve it in 5 mL of ethanol to obtain an ethanol solution of the ligand;
[0044] Weigh 20 mg of zinc nitrate and dissolve it in 5 mL of water to obtain an aqueous solution of zinc nitrate;
[0045] The aqueous solution of zinc nitrate was added to a 20 mL flat-headed test tube, 3 mL of ethanol was added along the wall, and then the ethanol solution of the ligand was slowly added. After standing for seven days, orange flake crystals were obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com