Compositions and Methods for Preventing or Treating Diseases, Conditions, or Processes Characterized by Aberrant Fibroblast Proliferation and Extracellular Matrix Deposition
A composition and characterization technology, which can be applied in drug combinations, urinary system diseases, cardiovascular system diseases, etc., can solve problems such as inability to compensate for MK2 loss, environmental pollution, antitrypsin deficiency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0826] Example 1. IC of MMI-0100 (YARAAARQARAKALARQLGVAA; SEQ ID NO: 1) 50 And specificity.
[0827] Use Millipore's IC 50 The Profiler Express service measures the IC of MK2 inhibition (MMI-0100; YARAAARQARAKALARQLGVAA (SEQ ID NO:1)) 50 (Half inhibition concentration) value. This quantitative determination measures how many inhibitors are needed to inhibit 50% of a given biological process or a component of that process (ie, enzymes, cells, or cell receptors) [IC 50 ]. Specifically, in these assays, if the kinase is not inhibited by the inhibitor peptide, the positively charged substrate is phosphorylated by the radiolabeled phosphate group from ATP. The positively charged substrate is then attracted to the negatively charged filter, quantified using a scintillation counter, and compared to a 100% active control.
[0828] Choose an ATP concentration with an apparent Km of ATP within 15 μM, because an ATP concentration close to the Km allows the kinase to have the same relative am...
Embodiment 2
[0842] Example 2. MMI-0100 (YARAAARQARAKALARQLGVAA; SEQ ID NO:
[0843] 1) Preparations and their functional equivalents
[0844] According to some embodiments, MMI-0100 (YARAAARQARAKALARQLGVAA; SEQ ID NO: 1) and its functional equivalent are formulated as a lyophilized powder via spray drying, micronization (eg, jet milling), or as a liquid formulation for spraying.
[0845] Spray drying
[0846] In some embodiments, taking the following factors into consideration, spray drying is used to prepare MMI-0100 (YARAAARQARAKALARQLGVAA; SEQ ID NO: 1) and its functional equivalents:
[0847] (a) Proteins and peptides are prone to denaturation—that is, they break into tertiary structure and sometimes break into secondary structure;
[0848] (b) Denaturation can be reversible or irreversible, and can be caused by various conditions, such as temperature increase, temperature decrease, extreme pH, addition of solvents, pressure, and shear denaturation (this applies to micronization);
[0849] (c) De...
Embodiment 3
[0856] Example 3. Mass production of MMI-0100 (YARAAARQARAKALARQLGVAA; SEQID NO:1) for continuous aerosol performance evaluation
[0857] Perform 2-3 spray drying runs under the defined process parameters described above to generate materials for aerosol performance evaluation.
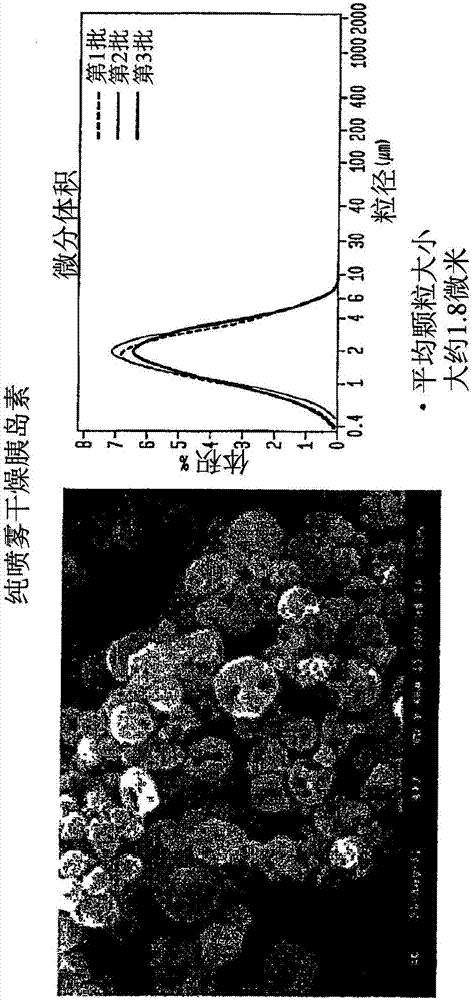
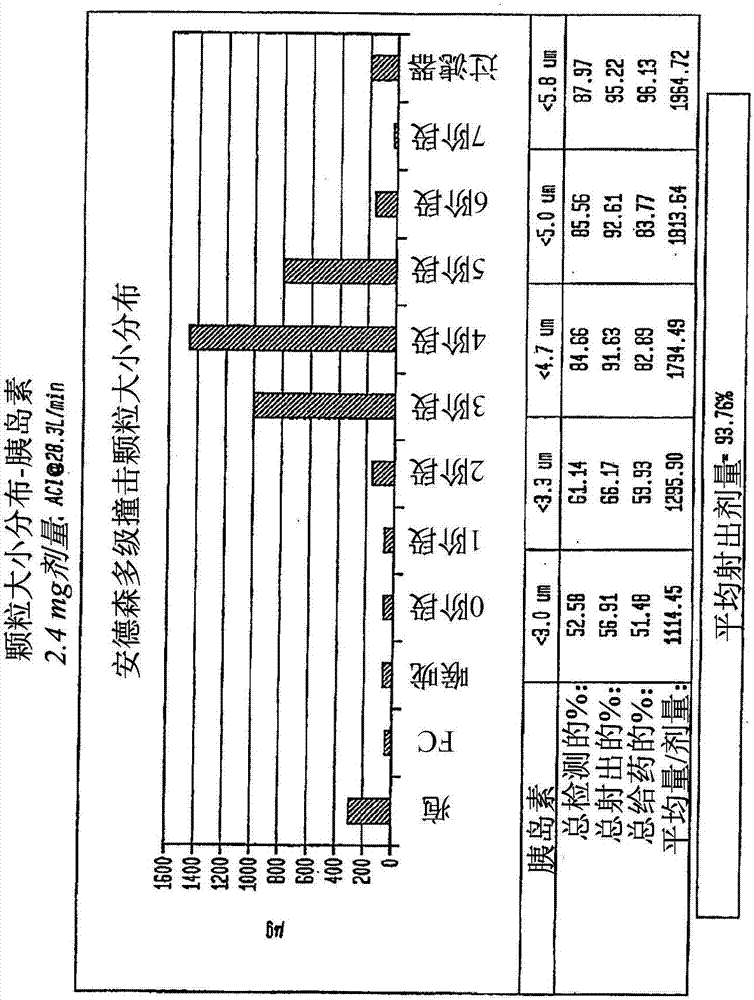
[0858] The spray-dried powder is well suited for delivery from an inhaler, for example, without limitation, a micro-dose inhaler. For both pure or co-spray dried blends, microdosing utilizes this formulation scheme to routinely achieve high shot doses, as well as both high fine particle fractions and doses. The exemplary aerosol properties of spray dried insulin are shown in figure 1 with 2 in.
[0859] Although dry micronization is the preferred powder production method for small molecules for pulmonary delivery, compared to spray drying, it is a pressure generation method that uses high shear forces. Because the use of high shear forces can lead to the fragmentation of proteins and peptides, dry powderin...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap