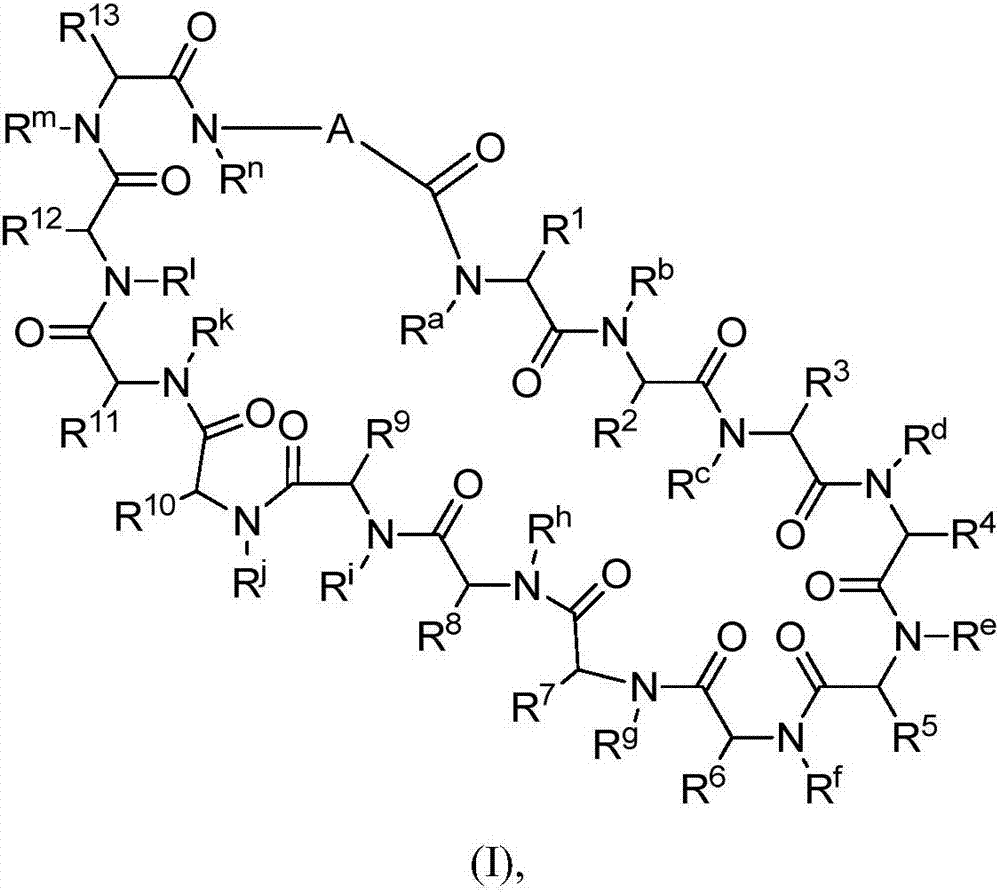
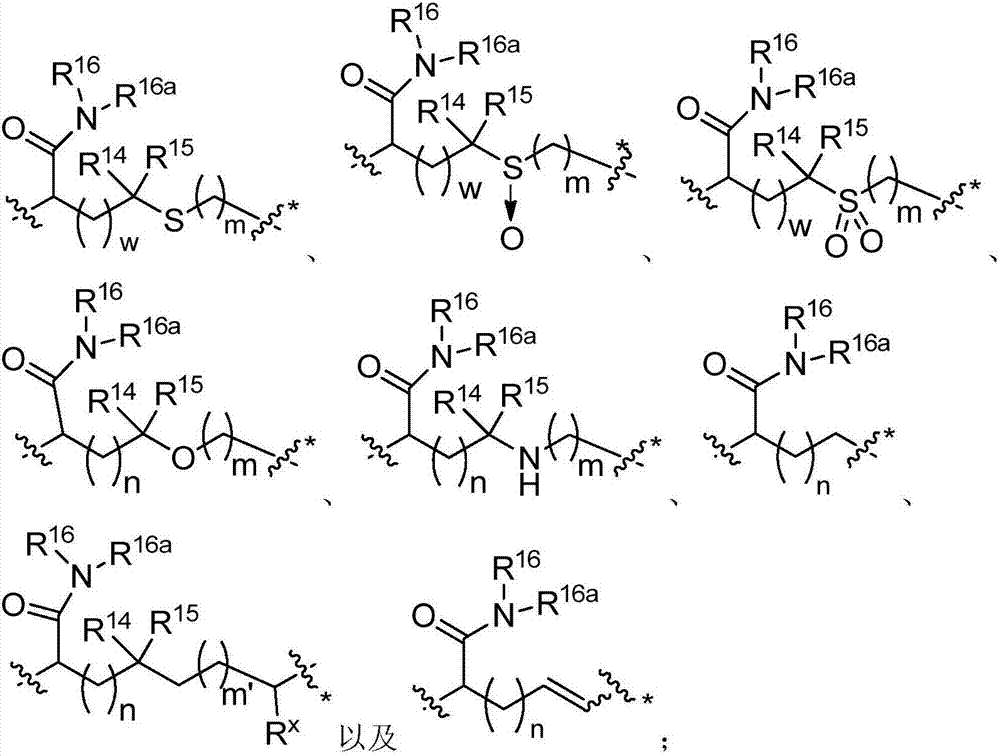
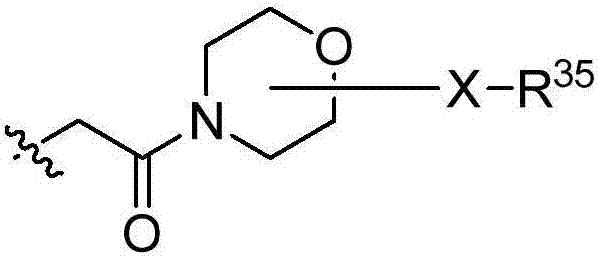
Macrocyclic peptides useful as immunomodulators
A medicinal salt and selected technology, applied in the field of macrocyclic peptides used as immunomodulators, can solve problems such as the lack of MYPPY motifs, and achieve the effect of improving various diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 0001
[0794] Example 0001 - Solid Phase Peptide Synthesis and Cyclization of Peptides
[0795] The procedures described in the following examples, in whole or in part, were used to synthesize the macrocyclic peptides in Tables 1, 2, 3, 4 and 5.
[0796] Scheme 1 – General synthetic method for thioether-cyclized peptides
[0797]
[0798] General protocol for solid-phase peptide synthesis and macrocyclization. Sieber amide resin (0.71mmol / g, 0.100mmol, 141mg ) with DMF (7mLx4min) swelling, every 30 seconds with N 2The airflow stirred slightly. Drain off the solvent and couple the first amino acid using the following method: wash the resin twice with 20% piperidine / DMF (5 mL) for 2.5 min each to remove the Fmoc group from the resin-loaded binding block, and every 30 sec Agitate gently with nitrogen stream. The resin was washed three times with DMF (5-8 mL and 1.5 min per wash). Then 2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)acetic acid (0.2 M in DMF, 0.5 mmol) was added, f...
preparation Embodiment 11001
[0854]
Embodiment 11001
[0855] The preparation of Example 11001 followed the general synthetic sequence described for the preparation of Example 0001 and consisted of the following general procedures: "Symphony Method A: Resin Swelling Procedure", "Symphony Method A: Standard Coupling Procedure", "Symphony Method A: Secondary Amine Coupling Protocol A", "Common Amino Acid Coupling Protocol", "Chloroacetyl Chloride Coupling Protocol", "Overall Deprotection Protocol A", and "Cyclization Protocol A". Modified Rink resin D was used in this synthesis. "
[0856] The crude material was purified by preparative LC / MS under the following conditions: Column: Waters XBridge C18, 30x100 mm, 5-μm particles; Mobile phase A: 5:95 acetonitrile:water + 10-mM ammonium acetate; Mobile phase B: 95:5 Acetonitrile: water + 10-mM ammonium acetate; gradient: 30-100% B over 25 minutes, then hold at 100% B for 5 minutes; flow rate: 30 mL / min. Fractions containing the desired product were combined and dried by centrifugal ev...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com