Methods for transduction and cell processing
A cell, axis-of-rotation technology, applied in the field of cell processing, capable of solving problems where available methods are not fully satisfactory
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0602] Example 1: Viral transduction of primary human T cells in a centrifuge chamber
[0603] This example demonstrates the transduction of isolated primary human T cells with a recombinant viral vector encoding a chimeric antigen receptor (CAR), wherein, according to the embodiments provided herein, the transduction takes place in a substantially stationary cylindrical centrifuge chamber. Start under centrifugal force. T cells were isolated from human apheresis samples by positive selection.
[0604] The resulting cells were activated using anti-CD3 / CD28 reagents. To initiate transduction, cells were incubated after activation with viral particles containing the viral vector genome encoding the anti-CD19 CAR under various conditions.
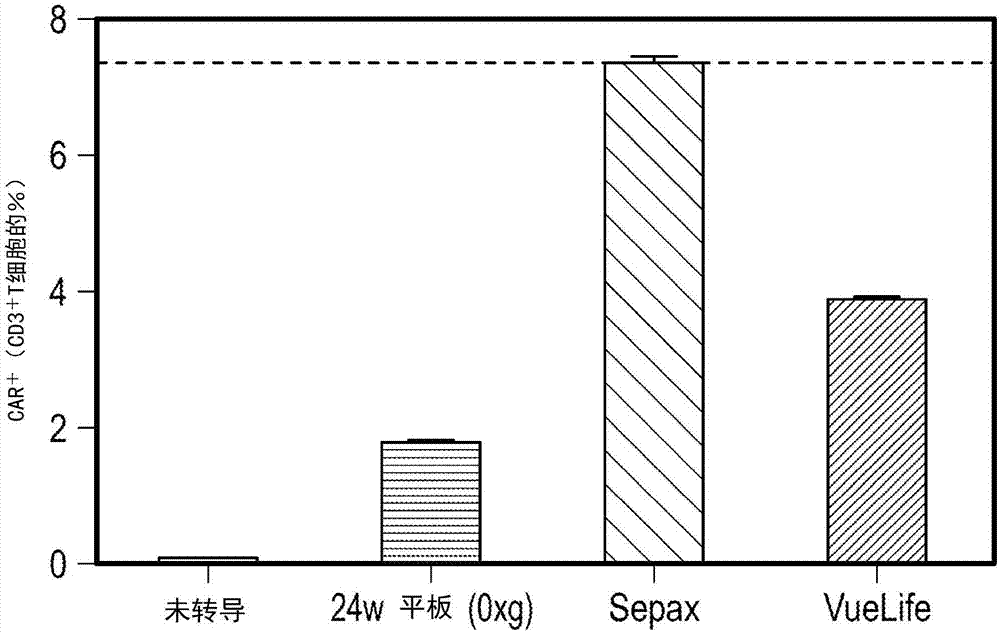
[0605] Under a set of conditions ("Sepax"), by Transduction was initiated by incubating cells centrifuged in the cavity of a centrifuge chamber (Biosafe SA, A200) in a 2-processing unit (Biosafe SA). 50mL contains 50×10 6 The liquid comp...
Embodiment 2
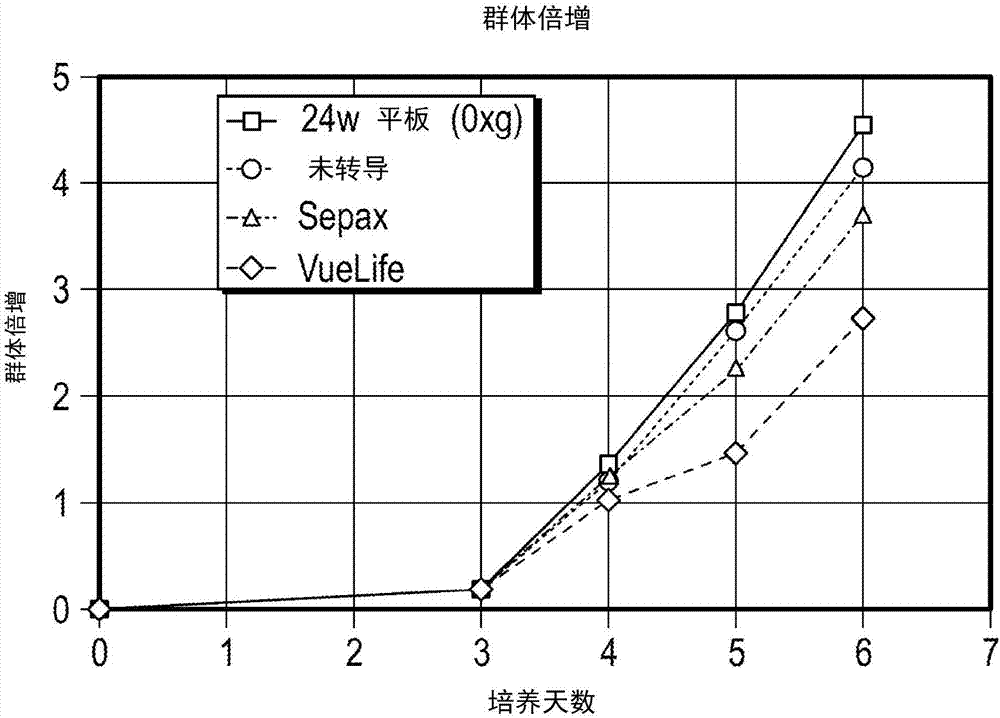
[0608] Example 2: Transduction of primary human T cells in centrifuge chambers with different ratios of liquid volume to surface area
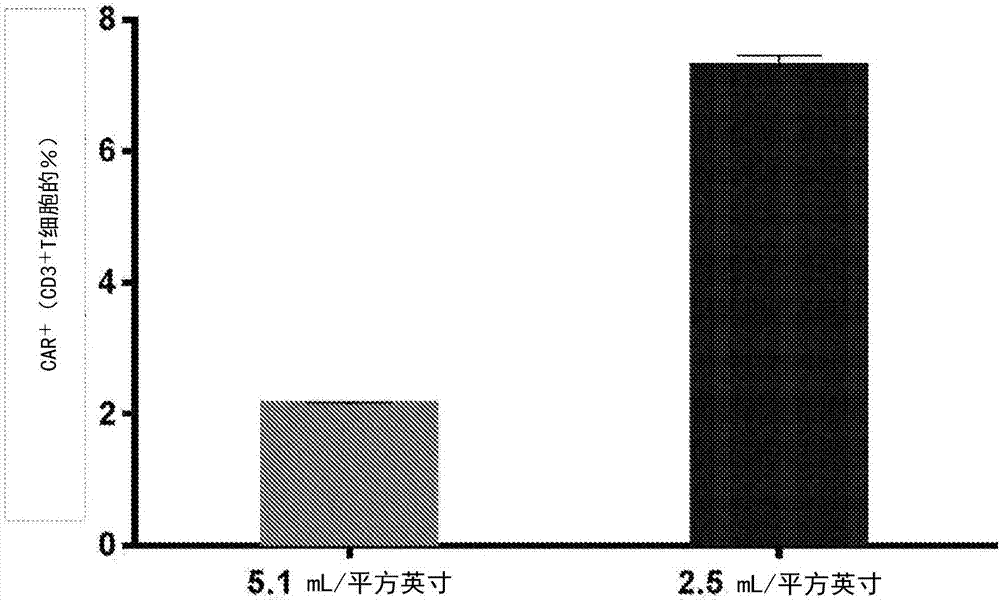
[0609] Transduction efficiency after initiation of transduction in the centrifuge chamber was assessed under various conditions using the same number of cells and virus infection units and different ratios of liquid volume to internal surface area of the centrifuge chamber cavity. Cells were generally prepared and stimulated as described in Example 1. All transductions were initiated using a 2:1 IU:cell ratio with 100×10 6 total number of cells.
[0610] For the first sample ("5.1mL / sq.in." means 5.1:1mL liquid / square inch internal cavity surface used in this condition), containing 100×10 6 A liquid volume of 100 mL of cells is combined with 100 mL of the liquid composition containing the viral vector particles. For the second sample ("2.5 mL / in2" means 2.5 mL of liquid per square inch of cavity surface used in this condition), 50 mL of t...
Embodiment 3
[0612] Example 3: Transduction of primary human T cells in a centrifuge chamber
[0613] Another study compared transduction efficiency under various conditions, including transduction in centrifugation chambers using various ratios of liquid volume to cavity surface area, according to embodiments of the methods provided. Human T cells were isolated from apheresis products and stimulated as described above.
[0614] After stimulation, the 80×10 6 Individual cells were incubated under different conditions, including transduction with a CAR-encoding viral vector. Polycations were included in all samples.
[0615] Conditions for initiating transduction in the centrifuge chamber, 80 x 10 6 cells were incubated with vector-containing virus at a rate of 2 IU virus per cell in the cavity of a centrifuge chamber. currently using 2 The treatment system was incubated while centrifuging the centrifuge chamber for 60 minutes with an RCF of approximately 600 g on the inner side wall ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com