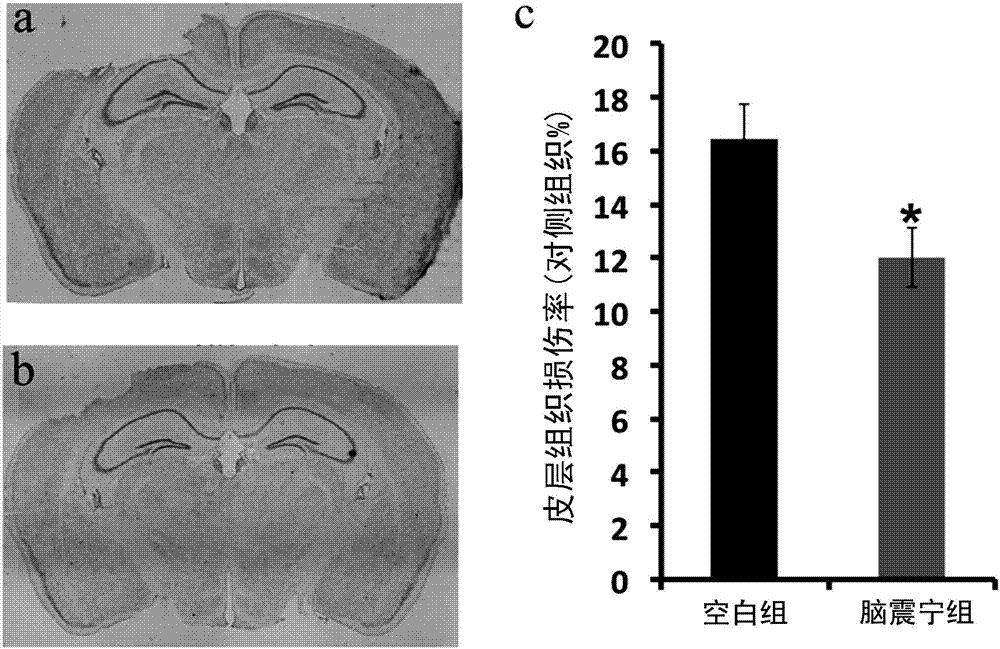
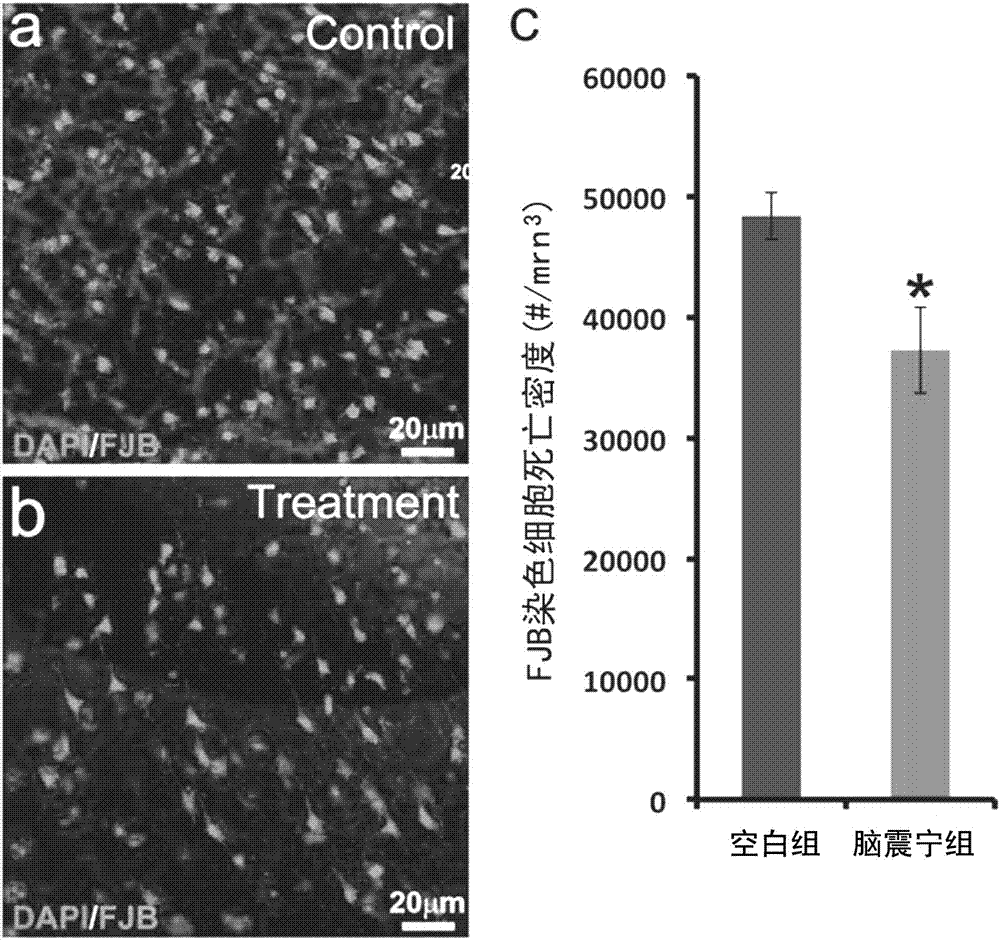
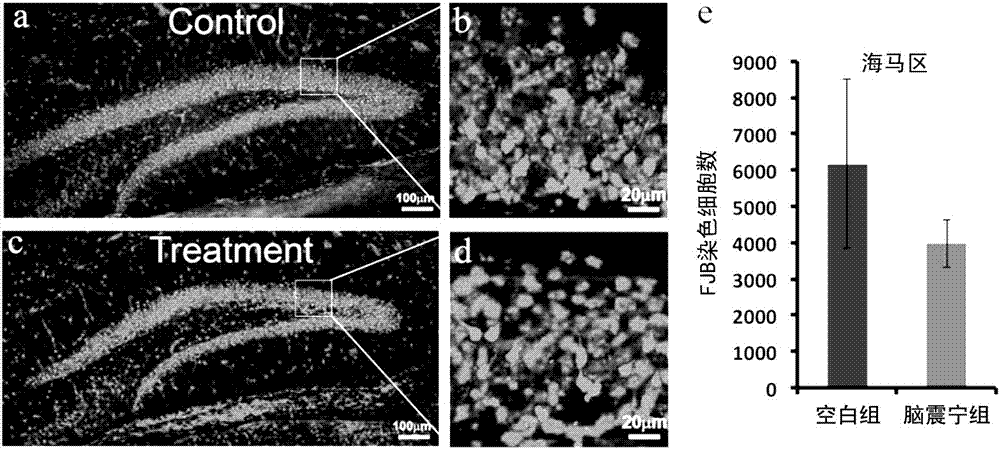
New pharmaceutical use of Naozhenning preparation
A technology of brain shock and preparations, applied in the direction of pill delivery, drug combination, pharmaceutical formula, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Embodiment 1: Brain Zhenning Extract
[0098] Brain Zhenning extract is a refined product made according to the prescription disclosed in the patent No. 931007410.
[0099] Prescription: Angelica 200g, Rehmannia glutinosa 200g, Moutan bark 150g, Chuanxiong 150g, Dilong 150g, Salvia miltiorrhiza 375g, Poria cocos 250g, tangerine peel 250g, Zhuru 250g, fried jujube kernel 250g, Baiziren 250g.
[0100] Preparation method: the above eleven flavors, angelica, moutan cortex, chuanxiong, and tangerine peel are distilled to extract volatile oil, then add other medicinal materials to extract with water, filter, and concentrate into a thick paste to obtain Zhenzhenning extract.
Embodiment 2
[0101] Embodiment 2: Naozhenning Granules
[0102] Sucrose-free Naozhenning granules were prepared according to standard WS3-B-3312-98-1.
[0103] Prescription: Angelica 200g, Rehmannia glutinosa 200g, Moutan bark 150g, Chuanxiong 150g, Dilong 150g, Salvia miltiorrhiza 375g, Poria cocos 250g, tangerine peel 250g, Zhuru 250g, fried jujube kernel 250g, Baiziren 250g.
[0104] The above eleven flavors, Angelica, Moutan Cortex, Chuanxiong, and Tangerine Peel are distilled to extract volatile oils, and the distilled aqueous solution is collected in a separate device; the dregs and the other seven flavors such as Salvia Miltiorrhiza are decocted twice, each time for 1.5 hours, and the decoctions are combined and filtered. Combine the filtrate with the above aqueous solution, concentrate to a clear paste with a relative density of 1.35-1.38 (55°C), add appropriate amount of sucrose and dextrin, granulate, dry, add the above-mentioned volatile oil, mix well, and make 1000g; Ningyouta...
Embodiment 3
[0105] Embodiment 3: Naozhenning Capsules
[0106] Prescription: Angelica 220g, Rehmannia glutinosa 210g, Moutan bark 160g, Chuanxiong 140g, Dilong 130g, Salvia miltiorrhiza 330g, Poria cocos 260g, tangerine peel 260g, Zhuru 260g, fried Suanzaoren 160g, Baiziren 160g.
[0107] Preparation method: the above eleven flavors, angelica, peony bark, chuanxiong, tangerine peel are distilled to extract volatile oil, and then add other medicinal materials for water extraction. After water extraction, use 80% ethanol to sink twice, concentrate into a thick paste, add appropriate amount of Mix the starch evenly, granulate, dry and granulate, add the obtained volatile oil, and stir evenly to prepare 200 Naozhenning capsules, each containing 0.4g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com