Pyrethroid pesticide hapten, preparation method and application thereof
A pyrethroid and hapten technology, applied in the field of food safety immunology detection, can solve the problems of unsuitable samples, high technical requirements, high cost, etc., and achieve the effects of good specificity, simple synthesis method and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Synthesis and identification of pyrethroid pesticide hapten
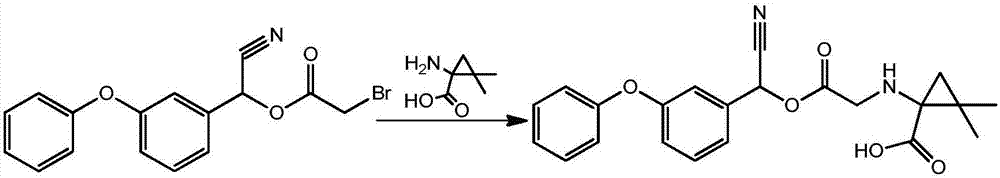
[0024] The synthetic route of pyrethroid pesticide hapten is attached figure 1 shown.
[0025] Take 1.0 g of [cyano-(3-phenoxy)-methyl] 2-bromoacetate, add N,N-dimethylformamide to dissolve, add 0.72 g of sodium bicarbonate, stir well, add 0.45g of 1-amino-2,2-dimethylcyclopropanecarboxylic acid, stirred at 60°C for 4h; stop the reaction, add water to extract with ethyl acetate, leave to separate layers, dry the organic phase with anhydrous sodium sulfate, evaporate to dryness, and Silica gel column, petroleum ether / ethyl acetate = 1:1 elution and separation, to obtain 1.1 g of cyclopropane carboxymethrin hapten, which is a pyrethroid pesticide hapten, with a yield of 97.35%.
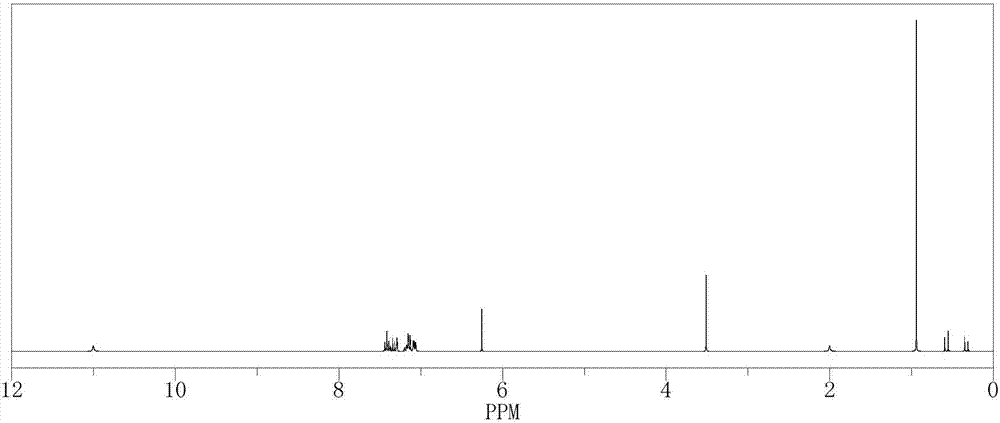
[0026] The above haptens were identified by H NMR spectroscopy, the results are shown in the attached figure 2 . 1 H-NMR (CDCl 3 ,300MHz) δ: 11.00(1H,s,COOH), 7.41(2H,dd,ArH), 7.17(1H,dd,ArH), 7.14(2H,dd,ArH), 7.08(2H,...
Embodiment 2
[0027] Example 2: Synthesis and identification of artificial antigens of pyrethroid pesticides
[0028] The carboxyl group of the pyrethroid pesticide hapten is used as the active site, and the immunogen is prepared by coupling with human serum albumin (HSA) by the mixed anhydride method; by coupling with ovalbumin (OVA) by the carbodiimide method, the preparation The package was originally.
[0029] 1. Preparation of immunogen by mixed anhydride method
[0030] Take 24mg of cyclopropanecarboxythrin hapten, add 0.5mL of dimethyl sulfoxide to dissolve, add 20μL of triethylamine, stir and mix well, add 20μL of isobutyl chloroformate, and stir for 30min to obtain hapten activation solution A; Add 0.1mol / L phosphate buffer solution to dissolve HSA 100mg to obtain solution B; add solution A dropwise to solution B, stir at room temperature for 4 hours, dialyze 0.01mol / L phosphate buffer solution for 3 days, change every day solution 3 times to obtain the immunogen, and store it at...
Embodiment 3
[0036] Example 3: Preparation, purification and identification of monoclonal antibodies
[0037] 1. Animal immunization
[0038]Take 10 healthy 6-8 week female Balb / c mice (divided into two groups, A and B, 5 in each group), and inject them subcutaneously at multiple points on the back of the neck after initial immunization with complete Freund's adjuvant emulsification, each small The mouse immunization dose was 200 μg of immunogen; after that, booster immunization was injected subcutaneously at multiple points on the back of the neck every two weeks, and Freund's incomplete adjuvant was used for emulsification; for the last immunization, physiological saline was used instead of Freund's incomplete adjuvant, and intraperitoneal injection was used. Dosage is the same as previous times. The specific immunization steps are shown in Table 1.
[0039] Table 1 Mouse immunization program
[0040]
[0041] 7 days after the third, fourth and booster immunizations, blood was coll...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com