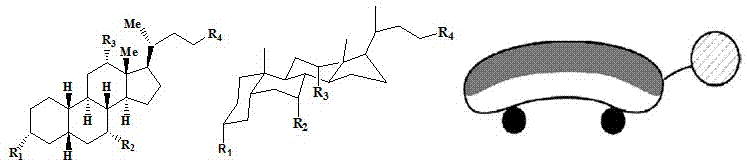
Sodium cholate-polymer nanometer composite drug carrier
A nanocomposite and polymer technology, applied in the fields of biomedicine and nanoscience, can solve the problem of unclear distinction between hydrophilic groups and hydrophobic parts, and achieve the effect of simple preparation method and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1. Preparation of a drug carrier with tunable polymer micelle morphology
[0030] 1. The mass ratio of amphiphilic block copolymer (polyethylene glycol-polylactic acid) to sodium deoxycholate is 100:5, weigh sodium deoxycholate and add it to 5ml of dimethyl sulfoxide (DMSO), stir for 1h, make it fully dissolved.
[0031] 2. Weigh 10 mg of doxorubicin hydrochloride (DOX HCL) and add it to the above solution, add 1 ml of triethylamine to remove the hydrochloride of DOX HCL, make it insoluble in water, stir overnight to make triethylamine and DOX fully react . 40 mg of polyethylene glycol-polylactic acid was added, and the polymer was dissolved by stirring.
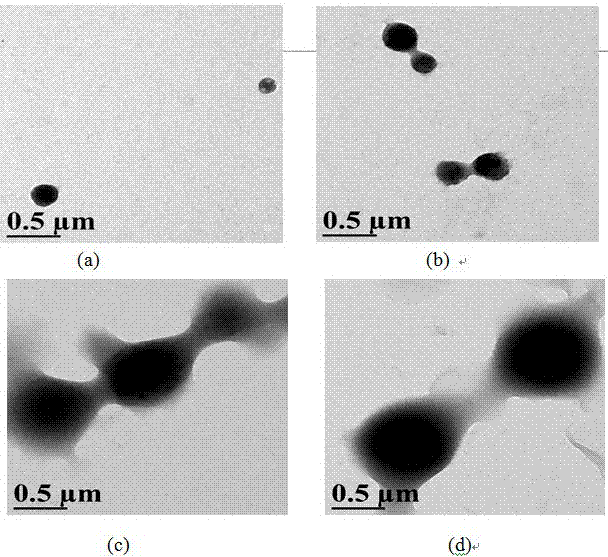
[0032] 3. Transfer the above mixed solution to a dialysis bag (molecular weight cut-off is 8-14kDa), dialyze in 500 mL of deionized water for 2 days, and change the water every 6h to remove the DMSO dialyzed. The compound drug carrier solution can be obtained. The transmission electron microscope observation resul...
Embodiment 2
[0034] 1. Preparation of a drug carrier with tunable polymer micelle morphology
[0035] 1. The mass ratio of block copolymer (polyethylene glycol-polylactic acid) to sodium deoxycholate is 100:10. Weigh sodium deoxycholate and add it to 5ml of dimethyl sulfoxide (DMSO), and stir for 1h to make it Fully dissolved.
[0036] 2. Weigh 10 mg of doxorubicin hydrochloride (DOX HCL) and add it to the above solution, add 1 ml of triethylamine to remove the hydrochloride of DOX HCL, make it insoluble in water, stir overnight to make triethylamine and DOX fully react . 40 mg of polyethylene glycol-polylactic acid was added, and the polymer was dissolved by stirring.
[0037] 3. Transfer the above mixed solution to a dialysis bag (molecular weight cut-off is 8-14kDa), dialyze in 500 mL of deionized water for 2 days, and change the water every 6h to remove the DMSO dialyzed. The compound drug carrier solution can be obtained.
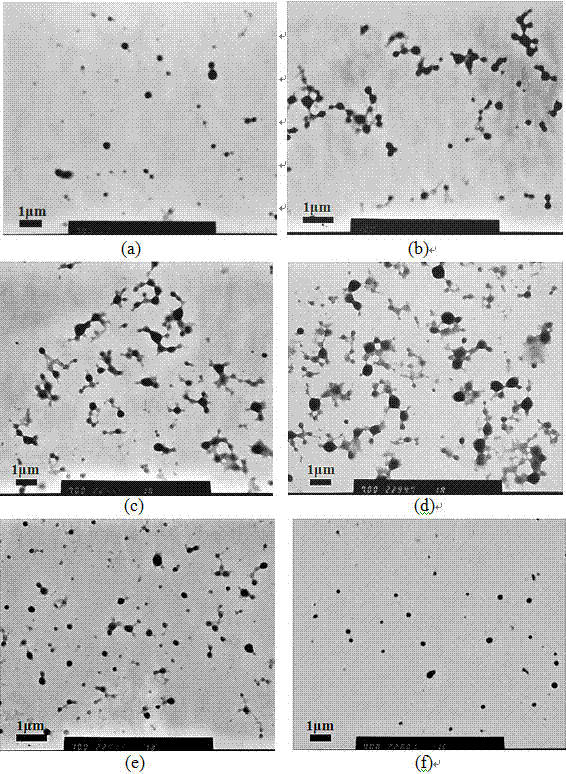
[0038] The particle size distribution results of the obta...
Embodiment 3
[0040] 1. Preparation of a drug carrier with tunable polymer micelle morphology
[0041] 1. The mass ratio of block copolymer (polyethylene glycol-polylactic acid) to sodium deoxycholate is 100:20, weigh sodium deoxycholate and add it to 5ml of dimethyl sulfoxide (DMSO) and stir for 1h to make it Fully dissolved.
[0042] 2. Weigh 10 mg of doxorubicin hydrochloride (DOX HCL) and add it to the above solution, add 1 ml of triethylamine to remove the hydrochloride of DOX HCL, make it insoluble in water, stir overnight to make triethylamine and DOX fully react . 40 mg of polyethylene glycol-polylactic acid was added, and the polymer was dissolved by stirring.
[0043]3. Transfer the above mixed solution to a dialysis bag (molecular weight cut-off is 8-14kDa), dialyze in 500 mL of deionized water for 2 days, and change the water every 6h to remove the DMSO dialyzed. The compound drug carrier solution can be obtained.
[0044] 2. In vitro release experiments
[0045] 2 mL of DO...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap