Lurasidone brain-targeted lipidosome injection and preparation method thereof
A technology targeting liposomes and injections, which is applied in liposome delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., and can solve problems such as low bioavailability, non-targeting, and poor patient compliance. To achieve the effects of prolonging cycle time, reducing dosage and improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
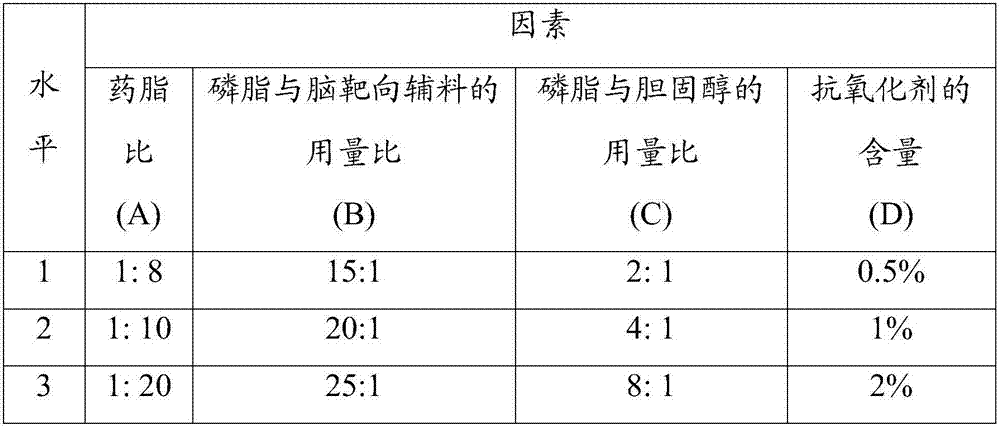
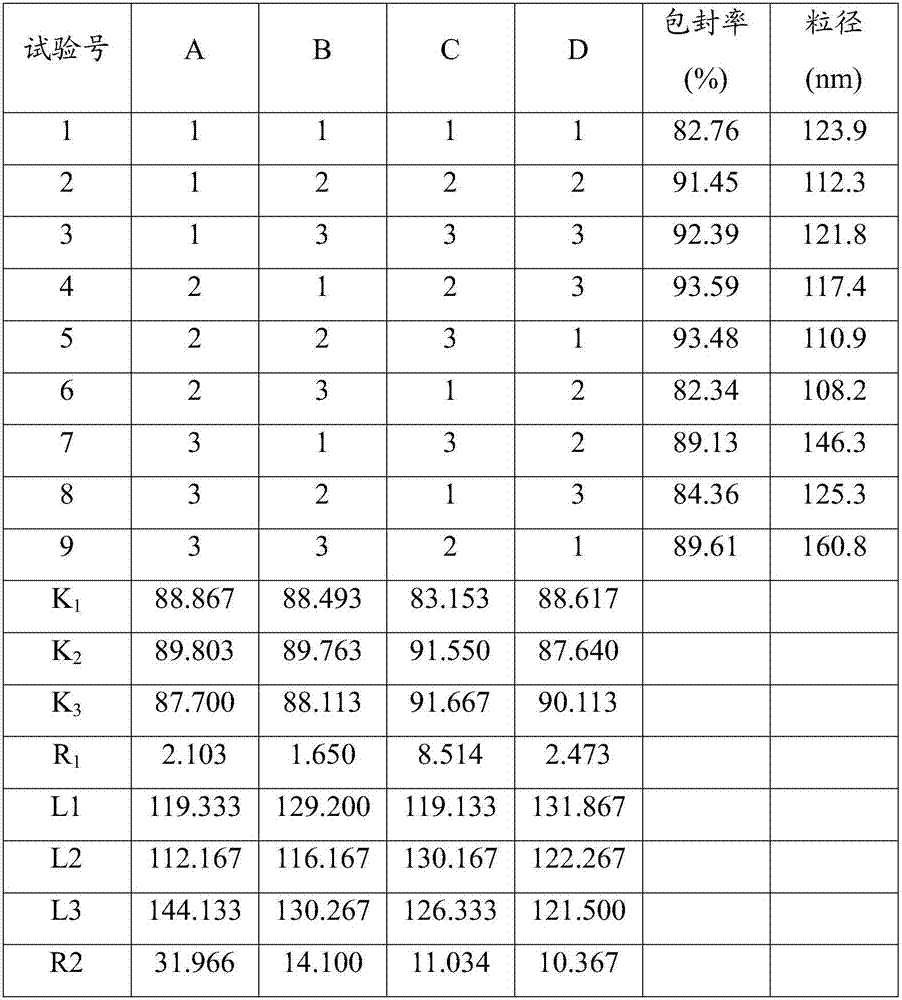
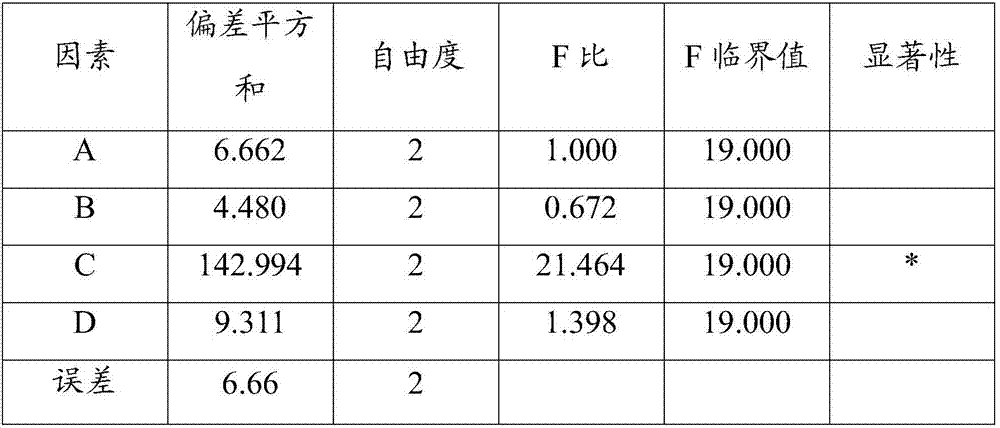
[0043] 1. Prescription ratio (100ml capacity)
[0044]
[0045]
[0046] 2. Preparation method
[0047] (1) Take (2,3-dioleoxypropyl)trimethylammonium chloride, cholesterol, (-)-epicatechin gallate and glucose-glycine-polyethylene glycol 1000 in the prescribed amount, Dissolve in 30ml of chloroform-methanol (2:1 by volume) mixed solvent, place in a constant temperature magnetic stirrer and stir until completely dissolved, then dissolve the prescribed amount of lurasidone in 0.05mol / L sodium glycinate solution, dropwise into the above-mentioned chloroform-methanol mixed solvent, after the dropwise addition, homogenize 3 times under 60MPa, homogenize 3 times under 160MPa to form a W / O type emulsion;
[0048] (2) Transfer the obtained W / O emulsion to a 300ml round-bottomed flask, and place it in a water bath temperature of 50°C for 55r·min -1 Rotary evaporation under reduced pressure to remove the organic solvent until a uniform phospholipid film is formed on the bottle wa...
Embodiment 2
[0054] 1. Prescription ratio (100ml capacity)
[0055]
[0056] 2. Preparation method
[0057] (1) Take (2,3-dioleoxypropyl)trimethylammonium chloride, cholesterol, (-)-epicatechin gallate and glucose-glycine-polyethylene glycol 1000 in the prescribed amount, Dissolve in 30ml of chloroform-methanol (2:1 by volume) mixed solvent, place in a constant temperature magnetic stirrer and stir until completely dissolved, then dissolve the prescribed amount of lurasidone in 0.05mol / L sodium glycinate solution, dropwise into the above-mentioned chloroform-methanol mixed solvent, after the dropwise addition, homogenize 3 times under 60MPa, homogenize 3 times under 160MPa to form a W / O type emulsion;
[0058] (2) Transfer the obtained W / O emulsion to a 300ml round-bottomed flask, and place it in a water bath temperature of 50°C for 55r·min -1 Rotary evaporation under reduced pressure to remove the organic solvent until a uniform phospholipid film is formed on the bottle wall of the ...
Embodiment 3
[0064] 1. Prescription ratio (100ml capacity)
[0065]
[0066] 2. Preparation method
[0067] (1) Take (2,3-dioleoxypropyl)trimethylammonium chloride, cholesterol, (-)-epicatechin gallate and glucose-glycine-polyethylene glycol 1000 in the prescribed amount, Dissolve in 30ml of chloroform-methanol (2:1 by volume) mixed solvent, place in a constant temperature magnetic stirrer and stir until completely dissolved, then dissolve the prescribed amount of lurasidone in 0.05mol / L sodium glycinate solution, dropwise into the above-mentioned chloroform-methanol mixed solvent, after the dropwise addition, homogenize 3 times under 60MPa, homogenize 3 times under 160MPa to form a W / O type emulsion;
[0068] (2) Transfer the obtained W / O emulsion to a 300ml round-bottomed flask, and place it in a water bath temperature of 50°C for 55r·min -1 Rotary evaporation under reduced pressure to remove the organic solvent until a uniform phospholipid film is formed on the bottle wall of the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| encapsulation rate | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap