A fluorescent functionalized carbonate, its preparation method and application, and fluorescent polycarbonate prepared therefrom
A technology of polycarbonate and carbonate, applied in chemical instruments and methods, organic chemistry, luminescent materials, etc., to achieve broad application prospects and research value, excellent AIE performance, and high yield effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
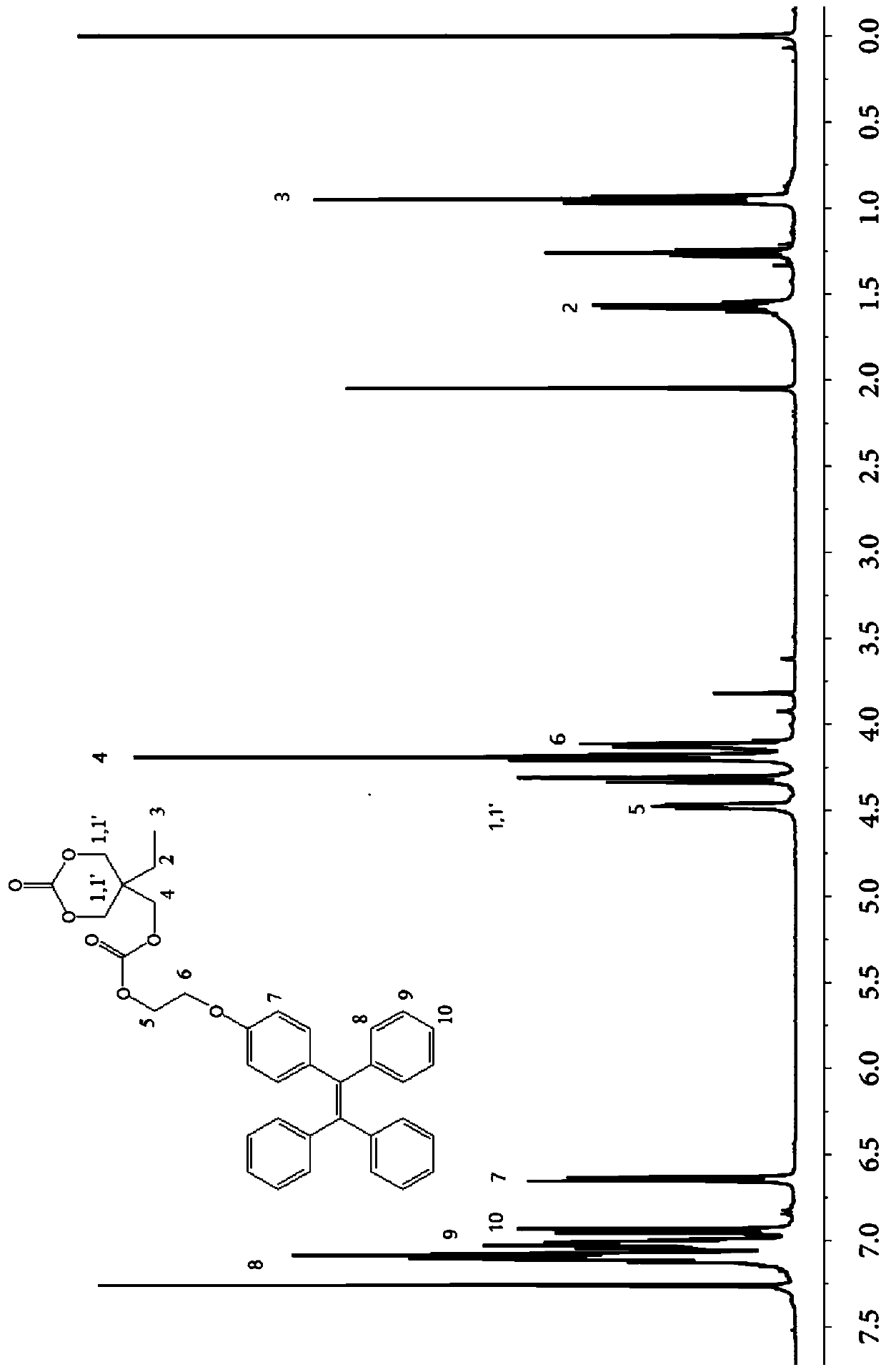
[0040] Example 1 Synthesis of fluorescent functionalized carbonate (TPETC)
[0041] Synthesis of 4-(1,2,2-triphenylvinyl)phenol
[0042]
[0043] Vacuumize the 250mL reaction bulb for three times, and add 150mL of tetrahydrofuran and 2.76g of activated zinc powder into the reaction bulb under the protection of argon. Then place the reaction bulb in an ice-water bath and stir for half an hour; then gradually add 0.24 mL of titanium tetrachloride using a syringe within half an hour to obtain a dark brown reaction solution. After the reaction solution returned to room temperature, it was slowly heated up and refluxed for 2 hours; after returning to room temperature again, 1.92 g of benzophenone and 2.10 g of 4-hydroxybenzophenone were added, and refluxed for 20 hours. The reaction was quenched with 100 mL of 10% potassium carbonate aqueous solution, and THF was removed by rotary evaporation. Finally, the product was extracted with dichloromethane, dried, concentrated, and se...
Embodiment 2
[0056] Example 2 AIE performance characterization of TPETC in solution environment
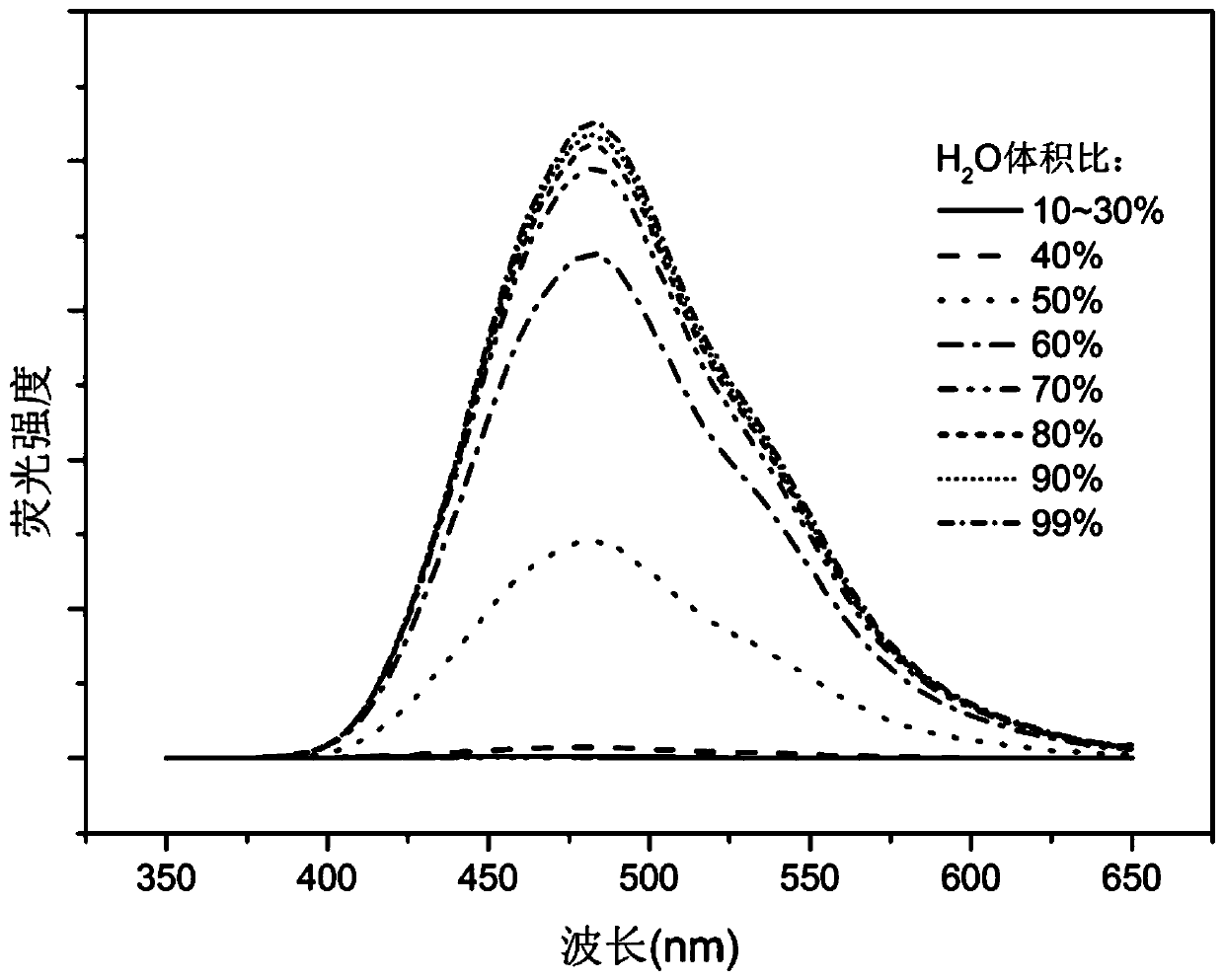
[0057] Water was slowly added to a solution of TPETC in dimethyl sulfoxide (DMSO). Prepare a series of DMSO-H with a concentration of 50 μM 2 O(100:0~1:99) mixture. image 3 Fluorescence spectra of a series of mixtures. When the proportion of water increases from 0% to 40%, the mixed system has almost no fluorescence; when the proportion of water reaches 50%, the fluorescence intensity of the mixed system increases sharply, and then the fluorescence intensity further increases with the increase of water content ,Such as Figure 4 shown. Figure 5 It is the change of the value of the fluorescence intensity at the maximum emission wavelength of 485nm in the emission spectrum.
Embodiment 3
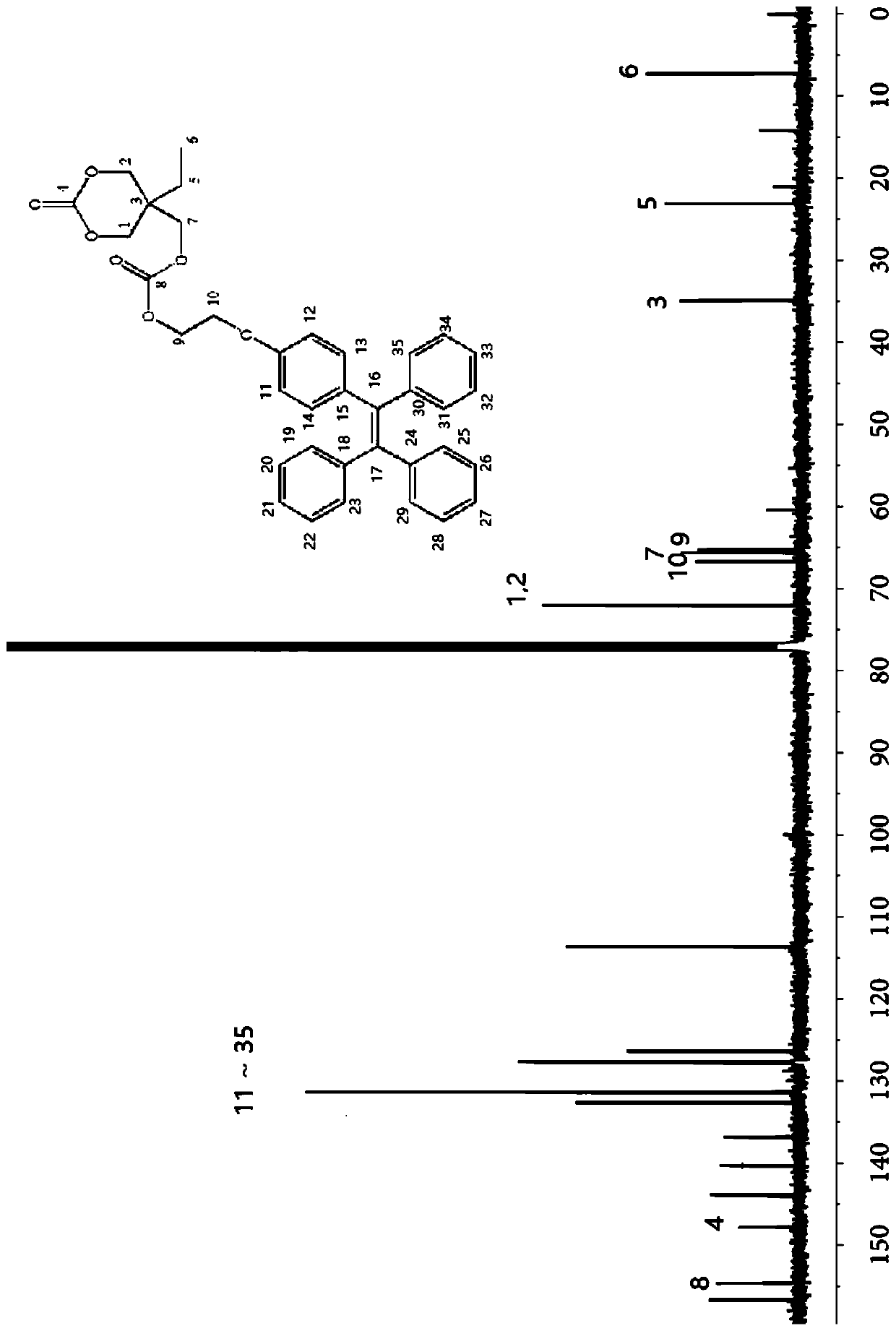
[0058] Embodiment 3 Preparation of TPETC amphiphilic block polymer
[0059] Under argon protection, mPEG2000 (0.03g), TPETC (0.18g) were added to the reaction flask. The reaction flask was then evacuated at 45 °C for 2.5 h. Then DBU (0.01mmol) and dichloromethane (0.7mL) were added into the reaction flask and reacted at room temperature for 72h. Glacial ether was precipitated to obtain fluorescent polycarbonate. Figure 4 It is the proton nuclear magnetic resonance spectrum of the product. Through the ratio of the peak area of the mPEG characteristic peak at 3.63ppm to the methylene characteristic peak on the carbonate at 4.10ppm, the degree of polymerization of TPETC is about 18.5, and the molecular weight of fluorescent polycarbonate is about 12700. . 1 H NMR (400MHz, CDCl 3 ,δ,ppm):7.20-6.95(m,15H),6.91(br,2H),6.66(br,2H),4.40(br,2H),4.10(br,8H),3.63(br,9.8H) ,1.50(br,2H),0.88(br,3H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com