A kind of glucosidase composition and the method for preparing icarigenin by enzymatic method
A technology of icariin and icariin, which is applied in the direction of biochemical equipment and methods, enzymes, hydrolytic enzymes, etc., can solve the problems of low product yield, long hydrolysis time, uncontrollable sequence, etc. High efficiency, short enzymatic hydrolysis time, and good substrate solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] 1.1 Synthesis of α-L-rhamnosidase gene derived from Aspergillus terreus CCF 3059, plasmid construction and preparation of recombinant enzyme
[0062] 1.1.1 Construction of recombinant plasmid pPICZαA-MRha
[0063] The whole gene was synthesized and the Aspergillus terreus CCF 3059 α-L-rhamnosidase gene (SEQ ID NO: 9) was optimized through yeast codon preference, and it was connected to the pPICZαA plasmid, and the obtained recombinant plasmid was named pPICZαA-MRha.
[0064] 1.1.2 Preparation of recombinant enzyme
[0065] The recombinant plasmid pPICZαA-MRha was extracted, linearized by Sac I, introduced into Pichiapastoris KM71H (Novagen) by electroporation, and positive clones were screened. After being inoculated in YPD medium for activation, transfer to BMGY medium to continue activation, and collect OD 600 The 2.0-3.0 bacterium was transferred into BMMY medium, and placed on a shaker at 30° C. at 180 rpm to induce the expression of α-L-rhamnosidase. Add sterile...
Embodiment 2
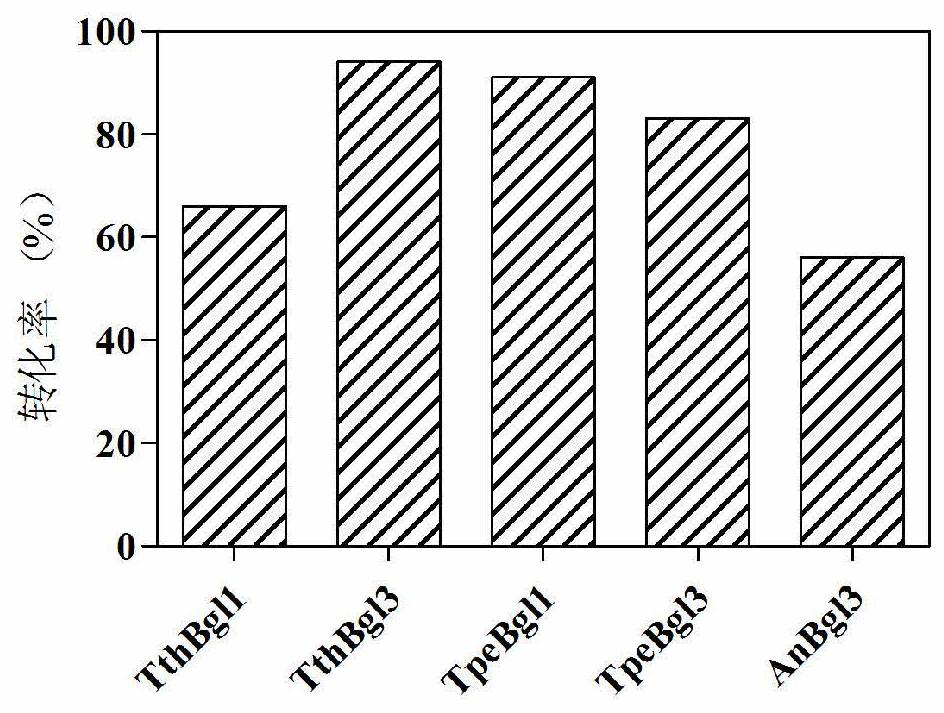
[0100] Example 2. Screening of β-glucosidase
[0101] 2.1 Cloning of β-glucosidase of GH3 family derived from Thermotoga thermarum DSM 5069 and preparation of recombinant enzyme
[0102] 2.1.1 Culture of Thermotoga thermarum DSM 5069
[0103] Thermotoga thermarum DSM 5069 was purchased from the DSMZ Culture Collection (www.dsmz.de). Number: DSM 5069. The medium formula is: 5g / L soluble starch, 1g / L yeast powder, 1.5g / L KH 2 PO 4 , 4.2g / LNa 2 HPO 4 x 12H 2 O, 3.4g / L NaCl, 1g / L MgSO 4 ×7H 2 O, 0.76g / L EDTA, 1mL / L trace elements, 0.5g / LNa 2 S·9H 2 O, 0.5g / L Cysteine HCl, 1mg / L resazurin, adjust the pH to 7.0, boil with nitrogen, remove oxygen, put the culture medium into an anaerobic bottle for sterilization under anaerobic conditions. Trace element (1000×) formula: FeCl 3 2.0g / L;H 3 BO 3 0.05g / L; ZnCl 2 0.05g / L; CuCl 2 2H 2 O 0.03g / L; MnCl 2 4H 2 O 0.05g / L; (NH 4 ) 2 MoO 4 0.05g / L; AlKSO 4 2H 2O 0.05g / L. ) was inoculated with a syringe according to...
Embodiment 3
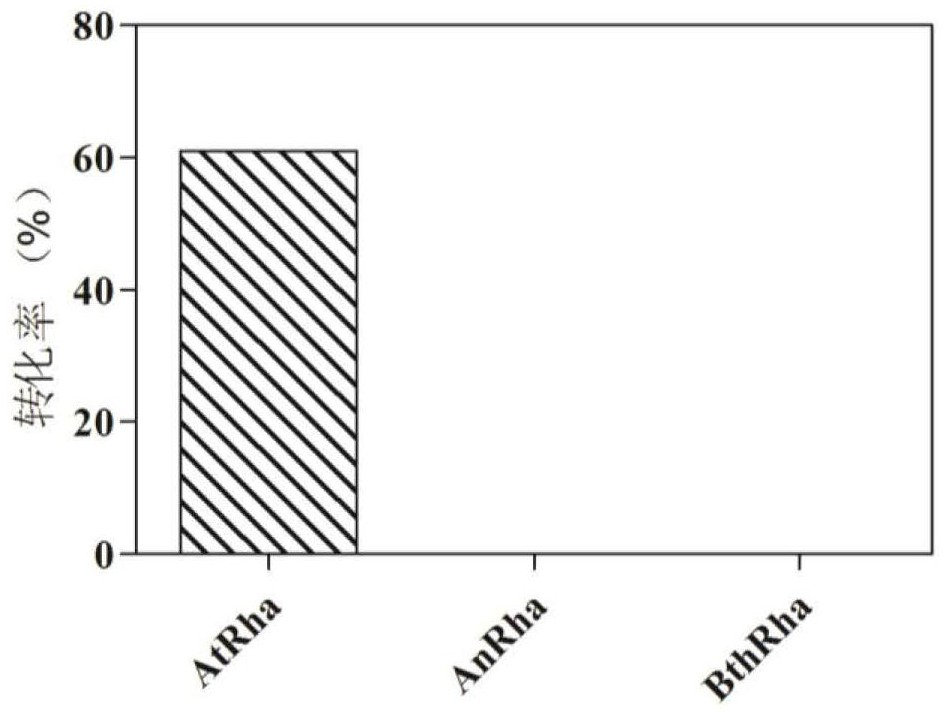
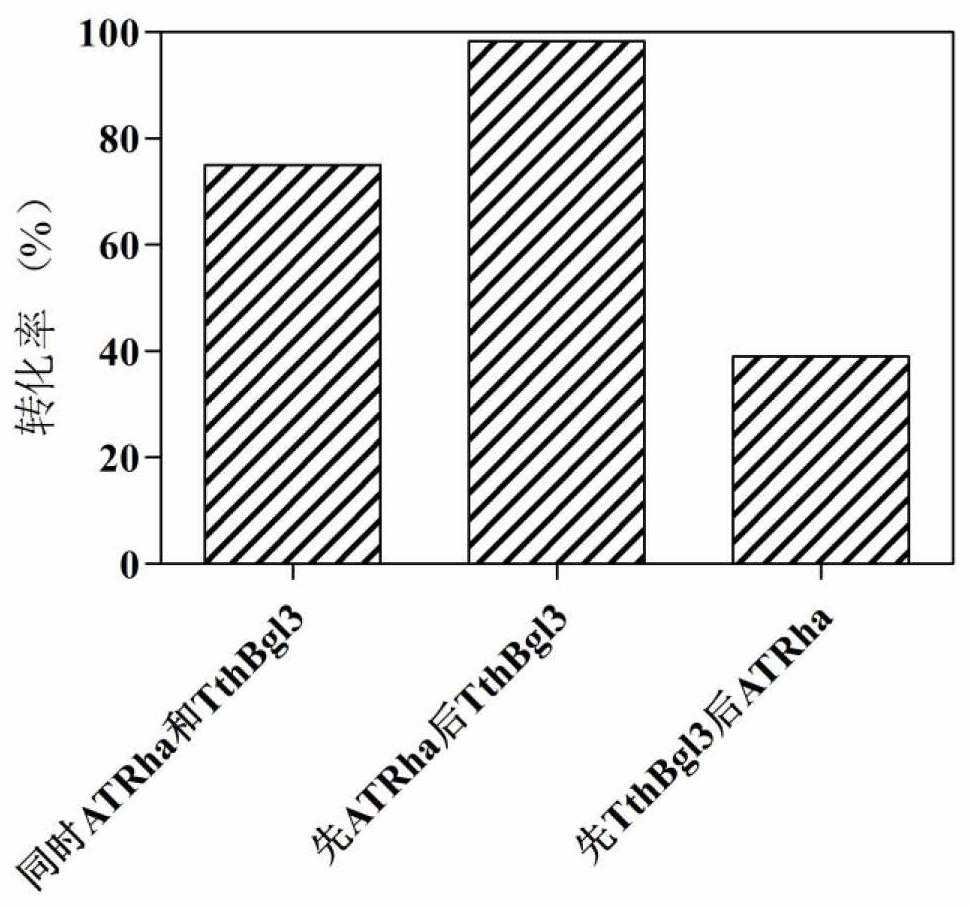
[0193] Example 3. α-L-rhamnosidase and β-glucosidase enzyme activity assay
[0194] Using p-nitrophenol-α-rhamnoside (pNP-R) as a substrate, the hydrolyzed p-nitrophenol had a color reaction with sodium carbonate, and the absorbance of the product was measured at a wavelength of 405nm. 100μL reaction system includes 75μL 100mM buffer solution with optimal pH, 20μL 5mM substrate, mix well and add 5μL diluted enzyme solution after preheating, react at the optimal temperature for 10min, then add 0.3mL 1M NaCO 3 Terminate the reaction, mix well and measure with a microplate reader under the condition of 405nm. At the same time, a control with enzyme solution without substrate and a control with substrate without enzyme solution were made.
[0195] Using p-nitrophenol-β-glucoside (pNP-G) as a substrate, the hydrolyzed p-nitrophenol had a color reaction with sodium carbonate, and the absorbance of the product was measured at a wavelength of 405nm. 100μL reaction system includes 90...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com