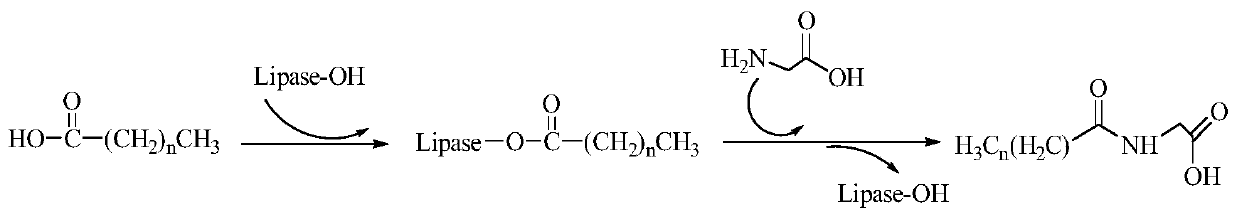Preparation method of lipid amino acid compound based on eutectic solvent
A low eutectic solvent, amino acid technology, applied in the preparation of organic compounds, the preparation of carboxylic acid amides, chemical instruments and methods, etc., can solve the problems of many by-products, low product synthesis efficiency, etc. Stability and activity, the effect of simplifying the purification process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Weigh 25mM lauric acid and 25mM glycine respectively, and add 10ml natural deep eutectic solvent (choline chloride-glycerol) and 12.5mg lipase CALB into a 25ml reaction bottle. Then the reaction bottle was sealed and placed in a constant temperature water bath shaker at 60 ° C and 2000 rpm for 48 h.
[0034] After the reaction, take out the reaction bottle, add a large amount of water, shake vigorously, and then centrifuge at 10,000rpm for 10min. After centrifugation, vacuum filter to obtain a white solid, add methanol, and recrystallize the crude product twice to obtain a pure product with a purity of 98%. . The product yield was 50.49%.
[0035] pass Figure 5 It can be seen from the mass spectrometry in the product that there is no dipeptide, a common by-product in the synthesis of lipoamino acid, which has a high product yield and fewer by-products.
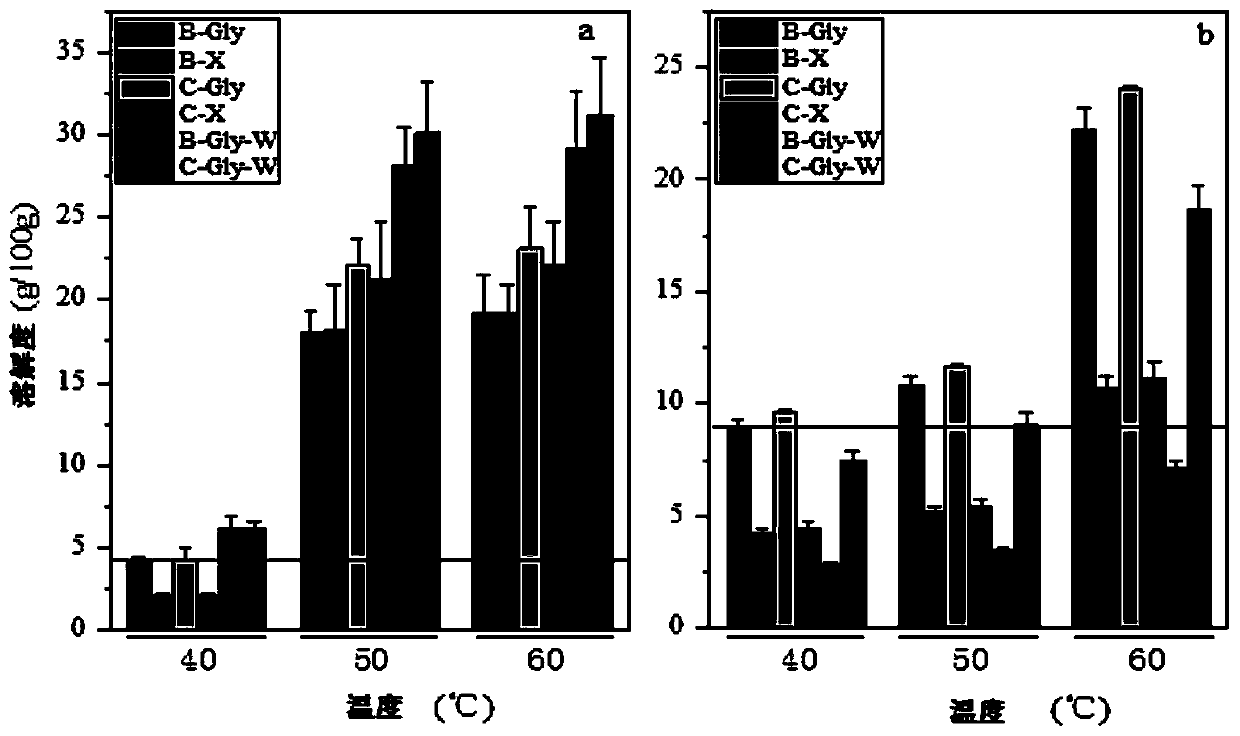
[0036] The cmc values of several products were measured by conductivity method, and lauroyl glycine was 0.0044m...
Embodiment 2
[0038] Weigh 25mM lauric acid and 25mM glycine respectively, and add 10ml natural deep eutectic solvent (betaine-glycerol) and 12.5mg lipase CALB into a 25ml reaction bottle. Then the reaction bottle was sealed and placed in a constant temperature water bath shaker at 60 ° C and 2000 rpm for 48 h.
[0039] After the reaction, take out the reaction bottle, add a large amount of water, shake vigorously, and then centrifuge at 10,000rpm for 10min. After centrifugation, vacuum filter to obtain a white solid, add methanol, and recrystallize the crude product twice to obtain a pure product with a purity of 98%. . The final yield of the product was 43.98%.
Embodiment 3
[0041]Weigh 25mM lauric acid and 25mM glycine respectively, and add 10ml natural deep eutectic solvent (betaine-xylitol) and 12.5mg lipase CALB into a 25ml reaction bottle. Then the reaction bottle was sealed and placed in a constant temperature water bath shaker at 60 ° C and 2000 rpm for 48 h.
[0042] After the reaction, take out the reaction bottle, add a large amount of water, shake vigorously, and then centrifuge at 10,000rpm for 10min. After centrifugation, vacuum filter to obtain a white solid, add methanol, and recrystallize the crude product twice to obtain a pure product with a purity of 98%. . The final yield of the product is 38.97%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Solubility | aaaaa | aaaaa |
| Solubility in water | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com