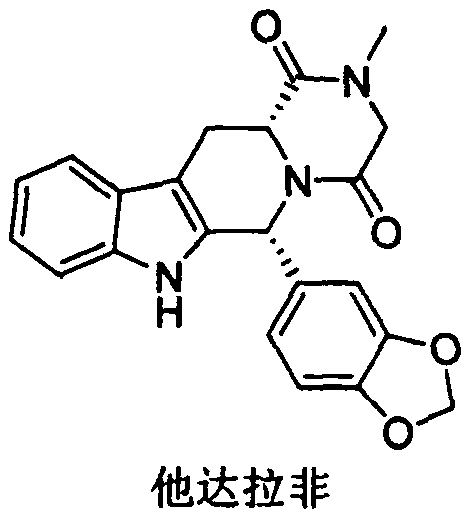
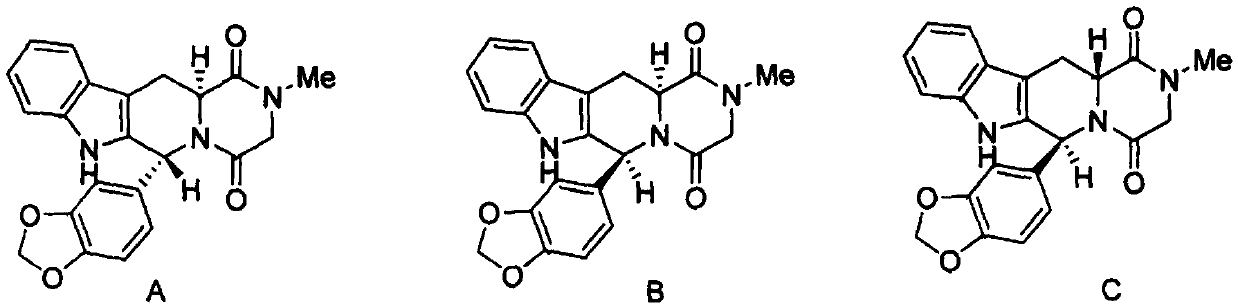
A kind of preparation method of tadalafil related substance f
A technology related to substances, tadalafil, applied in the field of medicine, can solve the problems of undiscovered synthesis methods and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
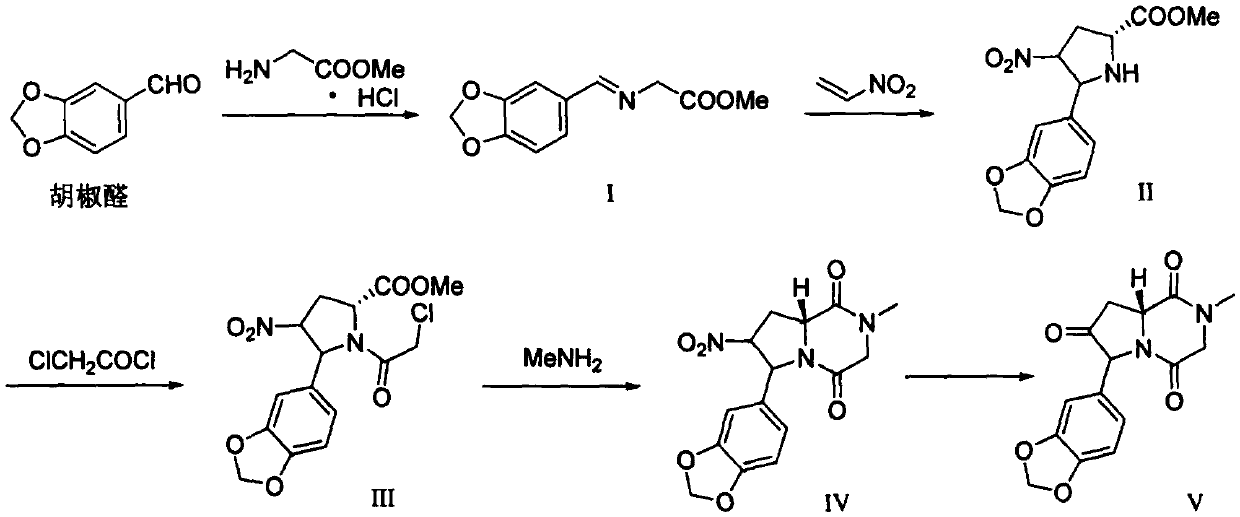
[0033] Add glycine methyl ester hydrochloride (37.67g, 300mmol), piperonal (64.86g, 432mmol), 452.00g methyl tert-butyl ether, 25.56g anhydrous sodium sulfate into a 1L reaction flask, and cool down to -5~5°C . Control the temperature at -5~5°C, add triethylamine (45.54g, 450mmol) dropwise, and react at room temperature 20~30°C for 48h after the dropwise completion. Suction filter, rinse with 226.00g of methyl tert-butyl ether, let the filtrate stand at 10~20°C for 1h, then stir at 5~10°C for 3h. After suction filtration, the solid was air-dried at 40°C to obtain compound I as a white solid (19.02 g, yield 28.7%).
[0034] Add compound I (19.00g, 85.9mmol), silver acetate (21.51g, 128.9mmol), triethylamine (10.70g, 105.7mmol) into a 1L reaction flask, add 232.40g of toluene, protect with nitrogen, and cool down to -5~ 5°C. Take 209.00g of toluene and cool it down to -10~0°C, mix it with nitroethylene (5.65g, 77.3mmol), and drop it into the above reaction flask. After dropp...
Embodiment 2
[0042] Add glycine methyl ester hydrochloride (37.67g, 300mmol), piperonal (64.86g, 432mmol), 452.00g tetrahydrofuran, and 25.56g anhydrous sodium sulfate into a 1L reaction flask, and cool down to -5~5°C. Control the temperature at -5~5°C, add pyridine (35.60g, 450mmol) dropwise, and react at room temperature 20~30°C for 48h. Suction filter, rinse with 226.00g of tetrahydrofuran, let the filtrate stand at 10~20°C for 1h, then stir at 5~10°C for 3h. After suction filtration, the solid was air-dried at 40°C to obtain compound I as a white solid (18.62 g, yield 28.0%).
[0043] Add compound I (18.00g, 81.4mmol), silver acetate (20.38g, 122.1mmol), pyridine (7.92g, 100.1mmol) into a 1L reaction flask, add 220.17g of toluene, protect with nitrogen, and cool down to 5~15°C. Take 198.00g of toluene and cool it down to -10~0°C, mix it with nitroethylene (5.13g, 73.2mmol), and drop it into the above reaction flask. After dropping, the temperature was controlled at 5~15°C for 6 hours...
Embodiment 3
[0051] Add glycine methyl ester hydrochloride (37.67g, 300mmol), piperonal (45.04g, 300mmol), 452.00g methyl tert-butyl ether, 25.56g anhydrous sodium sulfate into a 1L reaction flask, and cool down to -5~5°C . Control the temperature at -5~5°C, add triethylamine (45.54g, 450mmol) dropwise, and react for 48h at room temperature at 10~20°C. Suction filter, rinse with 226.00g of methyl tert-butyl ether, let the filtrate stand at 10~20°C for 1h, then stir at 5~10°C for 3h. After suction filtration, the solid was air-dried at 40°C to obtain compound I as a white solid (16.55 g, yield 24.9%).
[0052] Add compound I (14.00g, 63.3mmol), silver acetate (18.85g, 95.0mmol), triethylamine (7.69g, 76.0mmol) into a 1L reaction flask, add 232.40g of toluene, protect with nitrogen, and cool down to -5~ 5°C. Take 154.00g of toluene and cool it down to -10~0°C, mix it with nitroethylene (4.16g, 56.9mmol), and drop it into the above reaction flask. After dropping, the temperature was contr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com