Preparation method of cassava polysaccharide iron
A cassava polysaccharide and cassava starch technology, which is applied in the field of fine chemicals, can solve the problems of large amount of ethanol or methanol, high toxicity, and many by-product glucose, so as to reduce the generation of by-product glucose, improve the ability of comprehensive utilization, and high applicability Effect of oligosaccharide yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
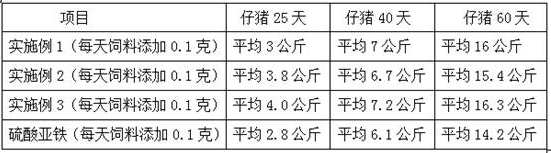
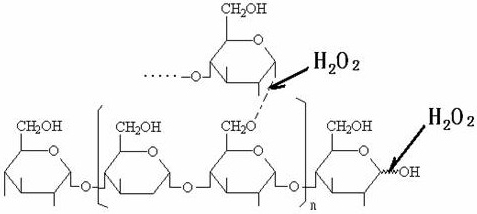
[0037] (1) Hydrogen peroxide oxidatively degrades tapioca starch to prepare suitable oligosaccharides: In a 200L enamel reaction kettle, start stirring, add 75.0 kg of water and 22.0 kg of tapioca starch, heat up to 60°C, and add 27.5% hydrogen peroxide 7.5 kg of solution, then heated up to 80°C, kept at a constant temperature, and carried out oxidation reaction for 60 minutes, when the reaction time was up, then added 29% sodium hydroxide seasoning solution with a pH value of 7.6, to obtain the suitable low-density compound with a weight average molecular weight of 4000-27000Da. polysaccharide solution.
[0038] (2) Complexation reaction: keep stirring, heat the prepared suitable oligosaccharide solution to 90°C, keep constant temperature, add 52 liters of 39.0% ferric chloride solution and 56 liters of 29.0% sodium hydroxide solution at the same time, Added within 3 hours. After adding the materials, adjust the pH value of the complexation reaction solution to 7.3 with 20% ...
Embodiment 2
[0043] ⑴ Hydrogen peroxide oxidatively degrades tapioca starch to prepare suitable oligosaccharides: In a 200L enamel reaction kettle, start stirring, add 80.0 kg of water and 22.0 kg of tapioca starch, heat up to 60°C, and add 27.5% hydrogen peroxide solution 8.5 kg, then raise the temperature to 88°C, keep the constant temperature, carry out the oxidation reaction for 45 minutes, when the reaction time is up, add 29% sodium hydroxide seasoning solution and the pH value is 7.2, that is, the suitable oligomer with a weight average molecular weight of 4000-27000Da sugar solution.
[0044] (2) Complexation reaction: keep stirring, heat the prepared suitable oligosaccharide solution to 95°C, keep the constant temperature, add 52 liters of 39.0% ferric chloride solution and 56 liters of 29.0% sodium hydroxide solution at the same time, in 2.5 hours Added. After adding the materials, adjust the pH value of the complexation reaction solution to 7.6 with 20% hydrochloric acid to obt...
Embodiment 3
[0049] ⑴ Hydrogen peroxide oxidatively degrades tapioca starch to prepare suitable oligosaccharides: In a 200L enamel reaction kettle, start stirring, add 85.0 kg of water and 22.0 kg of tapioca starch, heat up to 60°C, add 27.5% hydrogen peroxide solution 9.5 Then raise the temperature to 95°C, keep constant temperature, carry out the oxidation reaction for 30 minutes, when the reaction time is up, add 29% sodium hydroxide seasoning solution with a pH value of 8.0, and obtain suitable oligosaccharides with a weight average molecular weight of 4000-27000Da solution.
[0050] (2) Complexation reaction: keep stirring, heat the prepared suitable oligosaccharide solution to 85°C, keep a constant temperature, add 52 liters of 39.0% ferric chloride solution and 56 liters of 29.0% sodium hydroxide solution at the same time, in 2 Added within hours. After adding the materials, adjust the pH value of the complexation reaction solution to 8.0 with 20% hydrochloric acid to obtain the ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com